1. Background
Colorectal cancer (CRC) is the world’s largest cause of cancer-related death (1-3). Patients with stage I CRC have five-year survival rate of > 90%. However, patients with stage III/IV CRC have a survival rate decreased to 67%, and those with metastatic CRC have a survival rate of 8% (4). Although surgery and chemotherapy are the most common treatments for CRC, many CRC cases are resistant to chemotherapeutic agents, such as oxaliplatin and irinotecan, induced by compensatory activation of alternative pathways (5). Furthermore, the side effects of chemotherapy, such as stomach discomfort, exhaustion, vomiting, and diarrhea, can be severe (6-8). Therefore, it is very important to find anticancer compounds with less side effects.
Cancer cells could evade immunological destruction or even trigger T cell death by expressing programmed cell death-ligand 1 (PD-L1) on cell surface (9-11). Since the overexpression of PD-L1 in CRC has been associated with poor prognosis (12), PD-L1 has become a promising therapeutic target in cancer (13). Many signaling pathways and transcriptional factors have been implicated in controlling immune surveillance by modulating the expression of PD-L1. The expression of PD-L1 mediated by epidermal growth factor receptor (EGFR), signal transducer and activator of transcription 3 (STAT3), interleukin 6 (IL-6), and interferon gamma (IFN-γ) has been proven in a variety of cancers (14-19).
Trametes versicolor is widely used in East Asian traditional medicine. Typical components include polysaccharide Krestin (PSK) and polysaccharide peptide (PSP). They are commonly thought to offer anti-cancer properties, particularly in cases of breast, cervix, stomach, liver, lung, and prostate cancer (20, 21). Polysaccharide peptide and PSK are peptide moieties linked to beta-glucan backbones that have similar molecular weights of 100 kDa (21). Polysaccharide peptide has the potential to enhance overall survival in cancer patients, as well as clinical and life quality benefits with minimal side effects (22), which is far superior to chemotherapeutic agents. Polysaccharide peptide has been proven to have immune-modulatory properties in numerous studies, in addition to its therapeutic benefits on malignancies (23-27). Although there is little knowledge about the mechanisms of PSP, it is routinely used as a nutrient supplement for cancer patients (28).
2. Objectives
This study aimed to investigate the mechanism through which PSP can inhibit the growth of CRC and identify its pharmacological action to be utilized as an immune regulatory supplement.
3. Methods
3.1. Materials and Reagents
The antibodies of PD-L1, EGFR, and c-Jun were purchased from Sangon (Shanghai, China). P-EGFR (Tyr1068), STAT3, and p-STAT3 (Tyr705) antibodies were purchased from Cell Signaling Technology (MA, USA). Polysaccharide peptide powder was purchased from commercial resource (Winsor Healthcare, HK) and labelled with PSP 95% purity and LPS free. McCoy’s 5a medium, RPMI 1640 medium, and fetal bovine serum (FBS) were brought from Sangon (Shanghai, China). Penicillin, streptomycin, and MTT (3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyltetrazolium Bromide, A Tetrazole) were purchased from Invitrogen (California, USA). The primers were synthesized by Sangon (Shanghai, China).
3.2. Cell Culture
Human CRC cell lines HCT116 (CVCL0291) and HT29 (CVCL0320) were purchased from national cell bank (Shanghai). HT29 was cultured in McCoy’s 5a (containing 10% FBS and 1% penicillin and streptomycin). HCT116 was cultured in RPMI 1640 (containing 10% FBS and 1% penicillin and streptomycin). Cells were maintained in a humidified atmosphere of 95% air and 5% CO2 at 37 °C.
3.3. Cell Proliferation Assay
The cell proliferation and cytotoxicity of PSP to HCT116 and HT29 cell lines were tested by tetrazolium-based MTT method (29) in time end-point manners. Briefly, at the beginning, the single cells solution was (3,000 cells/well) allocated into each of 96 wells with 100 μL McCoy’s 5a and RPMI 1640 complete medium. The cells were treated with different concentrations of PSP (0, 1.5, 4.5, and 13.5 mg/mL) or etoposide (80 μg/mL) for 0, 2, 4, and 6 days. Subsequently, 10 μL of the MTT dye solution (5 mg/mL) was added to each well and incubated at 37°C for 4 hours. After incubation and removing the supernatants, 100 μL dimethylsulfoxide (DMSO) was added to each well. One hour after the addition of the solubilization solution, the content of the wells was mixed and read by the 96-well plate scanning spectrophotometer (μQuant, USA) and quantitative software (KC-junior, Bio-Tek Instruments, Inc. USA) at an absorbance of 595 nm for quantitative analysis.
3.4. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was isolated from HCT116 cells using TRIzol Reagent based on manufacturer’s protocol (Invitrogen, USA), and RNA (2 μg) was reverse transcribed by GoScript Reverse Transcription system (Promega, USA). We used the GoTaq quantitative polymerase chain reaction (qPCR) Master Mix (Promega, USA) to assess the expression of EGFR, PD-L1, STAT3, c-Jun, and NF-κB in HCT116 cells, and reduced glyceraldehyde-phosphate dehydrogenase (GAPDH) was used as an internal control. The quantitative real-time polymerase chain reaction (qRT-PCR) was accomplished in fast real-time PCR system (Applied Biosystems 7500, USA). The sense and antisense primers used in this experiment were listed in Table 1. The relative gene expression was calculated using the Delta CT Method (30). The experiments were performed in triplicate, and the results were presented as mean ± SD.
| Gene | Sequences | Length (bp) |
|---|---|---|
| Epidermal growth factor receptor | ||
| Forward primer | 5’-AGGCACGAGTAACAAGCTCAC-3’ | 177 |
| Reverse primer | 5’-ATGAGGACATAACCAGCCACC-3’ | |
| Programmed cell death-ligand 1 | ||
| Forward primer | 5’-TGGCATTTGCTGAACGCATTT-3’ | 120 |
| Reverse primer | 5’-TGCAGCCAGGTCTAATTGTTTT-3’ | |
| Activator of transcription 3 | ||
| Forward primer | 5’-CAGCAGCTTGACACACGGTA-3’ | 150 |
| Reverse primer | 5’-AAACACCAAAGTGGCATGTGA-3’ | |
| c-Jun | ||
| Forward primer | 5’-AACAGGTGGCACAGCTTAAAC-3’ | 77 |
| Reverse primer | 5’-CAACTGCTGCGTTAGCATGAG-3’ | |
| NF-κB | ||
| Forward primer | 5’-AACAGAGAGGATTTCGTTTCCG-3’ | 104 |
| Reverse primer | 5’-TTTGACCTGAGGGTAAGACTTCT-3’ | |
| GAPDH | ||
| Forward primer | 5’-GGAGCGAGATCCCTCCAAAAT-3’ | 197 |
| Reverse primer | 5’-GGCTGTTGTCATACTTCTCATGG-3’ |
The Sense and Antisense Primers for Target Genes Used in Quantitative Real-Time Polymerase Chain Reaction Analyses
3.5. Western Blotting
The cells were treated with different concentrations of PSP (0, 1.5, 4.5 and 13.5 mg/mL) or etoposide (80 μg/mL) for 48 hours. The cells were washed twice with ice cold phosphate buffered saline (PBS), and the cellular lysates were prepared in ice-cold lysis buffer (10 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM Na3VO4, 2 mM EDTA, 2 mM EGTA, 50 mM NaF, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS, 0.5 mM DL-dithiothreitol and proteinase inhibitor cocktails, Pierce, USA), and homogenized using a Sonic Dismembrator 100 (Fisher Scientific Inc., USA). The cell lysates protein concentration was determined by MicroBCA kit (Pierce, USA) and equal amounts of total proteins from different cell lysates loaded on SDS-PAGE (4 ~ 12%). Western blot analysis was done with target antibodies (EGFR, PD-L1, phosphorylated EGFR (Tyr1068), c-Jun, STAT3, phosphorylated STAT3 (Tyr705), GAPDH, and β-actin, diluted as 1:1000 with TBS-Tween-1% BSA) for 16 hours. Primary antibodies were visualized with ROX Dye goat-anti rabbit secondary antibodies using an Odyssey scanner (Li-cor biosciences, USA).
3.6. T-cells Kill Experiments
Peripheral blood mononuclear cells (PBMC) were purchased from commercial resource (TPCS, China) and activated by IL-2 (STEMCELL, Canada) and ImmunoCult Human CD3/CD28/CD2 T Cell Activator (STEMCELL, Canada). HCT116 cells were seeded (3 × 104 cells/well) in a 12-well plate with a glass coverslip on the bottom, either untreated or treated for 48 hours with PSP (4.5 mg/mL) or etoposide (20 μg/mL). Peripheral blood mononuclear cells were centrifuged at 200 g for 10 minutes. A final cell ratio of PBMC to HCT116 (5: 1) was achieved by adding PBMC (3 × 106 cells/well) to each 12 well of HCT116 cells, and filled with regular medium. Cell viability was determined using one step terminal-deoxynucleotidyl transferase-mediated nick-end labeling (TUNEL) Apoptosis Assay kit (Green Fluorescence, Beyotime, China) after co-culture for 24 hours.
3.7. Immunofluorescence
HCT116 cells were seeded in 6-well plates (3,000 cells/well), cultured on glass coverslips, and treated by PSP (4.5 mg/mL) or etoposide (80 μg/mL) for 48 hours. Cells were fixed with Parafix (Sigma, USA) for 15 minutes at 25°C, rehydrated in 1X PBS for three times (5 minutes each), and washed with 1X PBS. After blocking the specimen in blocking buffer for 60 minutes, we aspirated blocking solution and incubated the glass coverslips overnight at 4°C with diluted primary antibody (1: 50, Sangon, China). Then, we rinsed them three times in 1X PBS for 5 minutes each, incubated specimen with Goat-anti rabbit IgG/Alexa Fluor (1: 100, Bioss, China) for 1 hour, and protected them from light. Next, we rinsed the specimen and covered it with antifade mounting medium with DAPI (Beyotime, China). Finally, representative images were taken using a Leica SP8 laser scanning confocal microscope (Leica, Britain). Programmed cell death-ligand 1 antibody is the same as the western blot. Goat anti-rabbit IgG/Alexa Fluor was purchased from Bioss (Beijing, China). Antifade mounting medium DAPI was Beyotime (Shanghai, China). The positive rates of TUNEL per field and positive rates of PD-L1 per field were analyzed using ImageJ (National Institutes of Health, USA), and the figure was plotted using GraphPad Prism (GraphPad Software, USA). The experiments were performed in triplicate, and the results were presented as mean ± SD.
3.8. Statistical Analysis
The significant associations between cytotoxicity and western blotting band intensity, qRT-PCR ratio, and immunofluorescence intensity of each cell were determined by t-test with Prism software (GraphPad Software, Inc. San Diego, CA). The fold change of target proteins band intensity was normalized with GAPDH or actin, and then compared with untreated cells. All the experimental data were generated by at least three independently repeated experiments and expressed as mean ± SD. Significance level of the test was considered as 5% (P-value ≤ 0.05).
4. Results
4.1. Polysaccharide Peptide Inhibited Colorectal Cancer Cells Proliferation
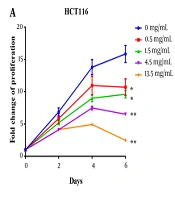
Polysaccharide peptide inhibited CRC cell proliferation of CRC cells (HCT116 and HT29) in a dose-dependent manner (from 0.5 to 13.5 mg/mL). After treating with PSP, the proliferation of both HCT116 and HT29 was inhibited, and HCT116 was shown to be more sensitive than HT29. After six days of incubation with 13.5 mg/mL of PSP, the suppression ratio increased to the highest level, with a 6.4-fold increase over untreated cells (Figure 1A). Polysaccharide peptide did not inhibit the growth of HT29 cells for the first two days, but by day four, it demonstrated the ability to suppress the tumor (Figure 1B). Polysaccharide peptide at 1.5 mg/mL suppressed CRC cell proliferation more effectively. Polysaccharide peptide showed tumor-suppressing effects on CRC cell lines. The HCT116 cell line was chosen to examine the mechanism of PSP inhibition due to its higher sensitive response to PSP. The dose of 4.5 mg/mL PSP was chosen for the following experiment because the cell sensitivity to inhibition was similar to the median among these doses.
Polysaccharide peptide inhibited proliferation of colorectal cancer cells. A, Polysaccharide peptide (0.5, 1.5, 4.5 and 13.5 mg/mL) treatment inhibited proliferation of HCT116 cell; and B, HT29 cells in a six-day PSP treatment for different time (0, 2, 4, and 6 days) was measured with MTT assay. Fold change means that each group was normalized with OD value of day 0. Results are presented as mean ± SD. * P < 0.05, ** P < 0.01, *** P < 0.001, t-test.
4.2. Polysaccharide Peptide Down-Regulated Expression of epidermal Growth Factor Receptor and Programmed Cell Death-Ligand 1 in HCT116 and HT29
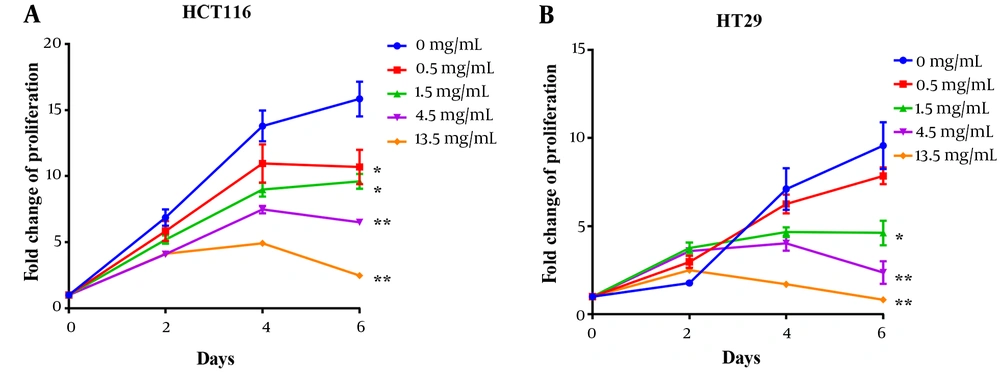
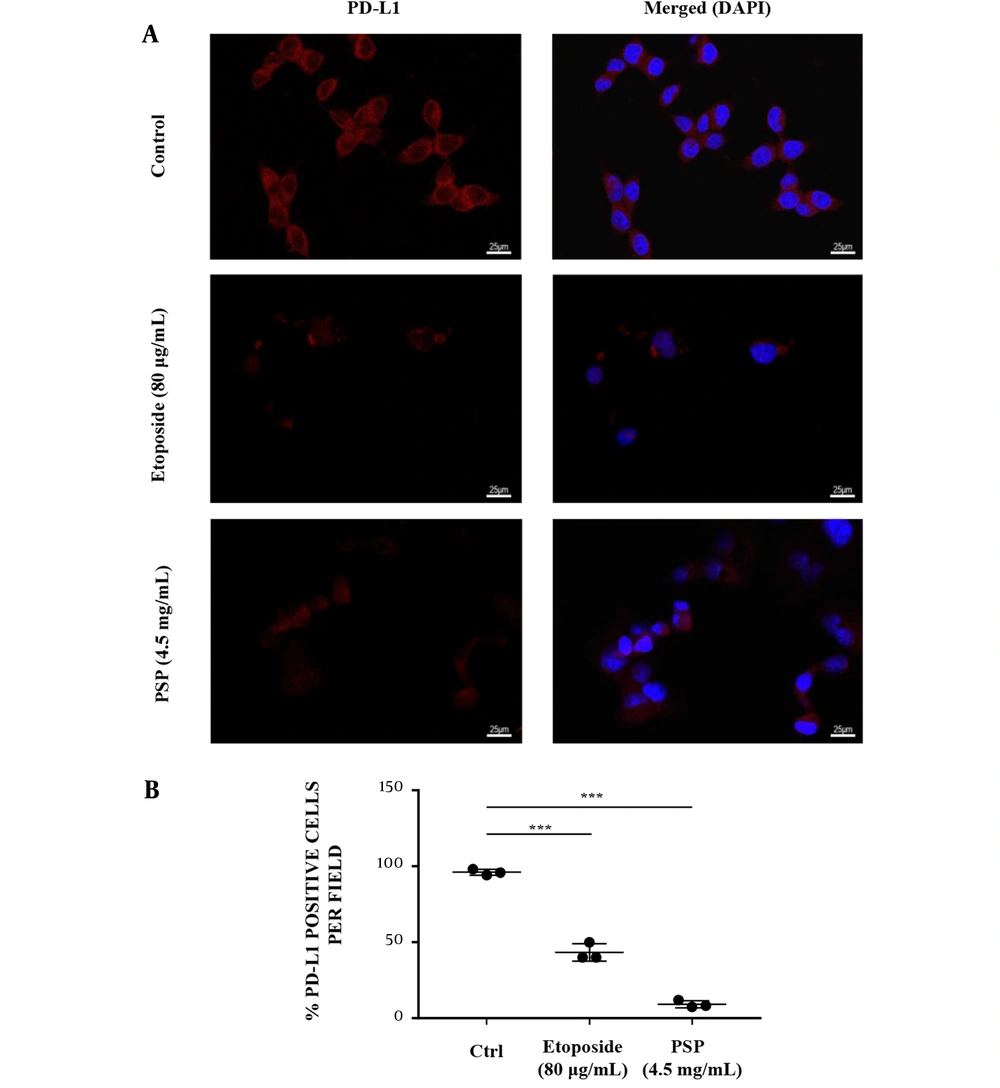
HCT116 and HT29 cells were treated with different concentrations of PSP for 48 hours to evaluate the impact on EGFR and PD-L1 expression by qRT-PCR and western blotting. As demonstrated by western blotting results, PSP significantly inhibited the expression of EGFR and PD-L1 at three distinct concentrations on both cancer cells (Figure 2A and F). Polysaccharide peptide (13.5 mg/mL) was far more effective than Etoposide in down-regulating the expression of EGFR and PD-L1. In addition, the gene expression of EGFR and PD-L1 impaired by PSP was confirmed by qRT-PCR results (Figure 3A). Furthermore, as PD-L1 is overexpressed in HCT116 cell membrane (31), the expression of PD-L1 impaired by PSP was tested by immunofluorescence analysis. Polysaccharide peptide treatment significantly reduced the PD-L1 expression in HCT116 membrane (Figure 4A and B). It is suggested that PSP works as a potential PD-L1 regulator on CRC cells. Overall, these results indicated that PSP reduced the expression of EGFR and PD-L1 protein and mRNA levels in HCT116 and HT29 cells.
Polysaccharide peptide down-regulated the expression of epidermal growth factor receptor and programmed cell death-ligand 1 in colorectal cancer cells. A, Western blot analysis expression of EGFR and PD-L1 in HT 29; and B, HCT116 cells after PSP (0.5, 1.5, 4.5 and 13.5 mg/mL, for 2 days) treatment; C - G, using ImageJ for quantitative analysis of the intensity of the western blot bands. The fold change of target proteins band intensity was normalized with GAPDH and compared with untreated cells. Results are presented as mean ± SD. * P < 0.05, ** P < 0.01, *** P < 0.001, t-test.
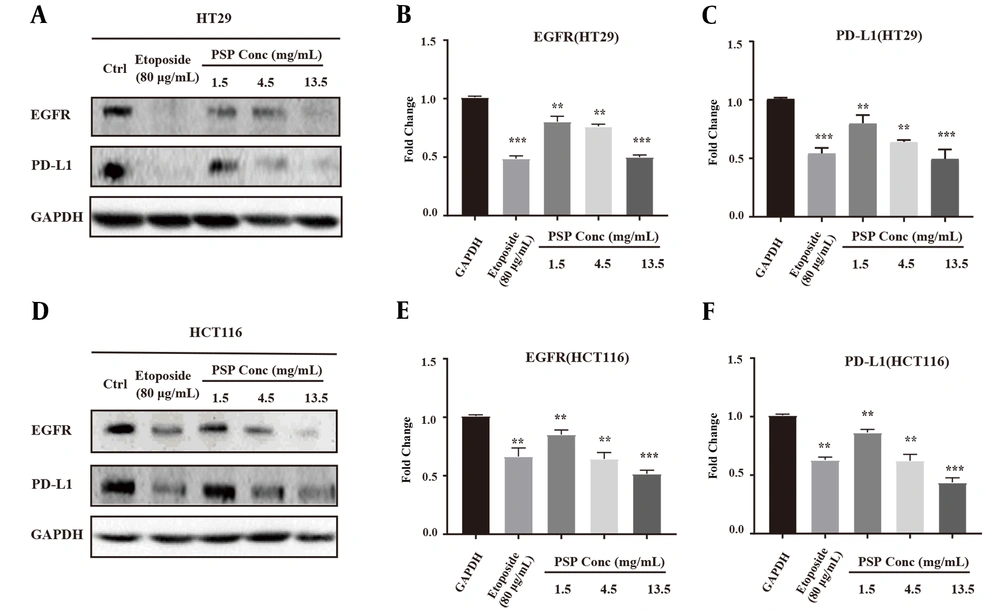
Polysaccharide peptide treatment down-regulated the expression of epidermal growth factor receptor and key related signaling molecules in HCT116 Cells. A, HCT116 cells were treated with PSP (4.5 mg/mL, 48 h) and the mRNA was extracted for quantitative real-time polymerase chain reaction analysis. The fold change of gene expression level was normalized with GAPDH and compared with untreated cells; B - F, HCT116 was treated with gradient concentration of PSP (0.5, 1.5, 4.5 and 13.5 mg/mL, for 2 days), and the cell lysate protein was collected for western blot, and the key signal molecules were detected (c-Jun, activator of transcription 3, p-STAT3 (Try705), p-EGFR (Tyr1068)). The fold change of target proteins band intensity was normalized with GAPDH or actin, and then compared with untreated cells. Results are presented as mean ± SD. * P < 0.05, ** P < 0.01, *** P < 0.001, t-test.
Immunofluorescence imaging analyzed the effect of polysaccharide peptide inhibition on programmed cell death-ligand 1 expression. HCT116 cells were treated with Etoposide (80 μg/mL) or PSP (4.5 mg/mL) for 48 hours and stained with labelled PD-L1 antibody (red) and DAPI nuclear stain (blue). The experiments were repeated three times to demonstrate consistency of the assay. (B) The mean of positive rates of PD-L1 per field in each view with PSP or Etoposide treatment compared to the untreated control. * P < 0.05, ** P < 0.01, *** P < 0.001, t-test.
4.3. Polysaccharide Peptide Impaired Key Regulators of Epidermal Growth Factor Receptor Signaling Pathway in HCT116
The gene expression of key regulators in HCT116, c-Jun, STAT3, and NF-κB were down-regulated by PSP treatment, which are involved in the expression and function of PD-L1 in many cancers (Figure 3A). The inhibitory effect of PSP was validated by western blot, and levels of c-Jun, STAT3, and p-STAT3 (Tyr705) were significantly decreased (Figure 3D - F). Overall, these results indicated that PSP reduced the expression of EGFR and PD-L1 protein and mRNA levels in HCT116. Also, PSP decreased site-specific phosphorylation level and hindered EGFR expression. Furthermore, PSP treatment reduced expression and phosphorylation of intracellular EGFR signaling cascade intermediates, such as EGFR, p-EGFR (Tyr1068), STAT3 and p-STAT3 (Tyr705), and c-Jun (Figure 3B - F). The downstream signaling molecules of EGFR pathway were regulated at varied levels.
4.4. Polysaccharide Peptide Pre-treatment Triggered Immunogenic Cell Death (ICD) of HCT116
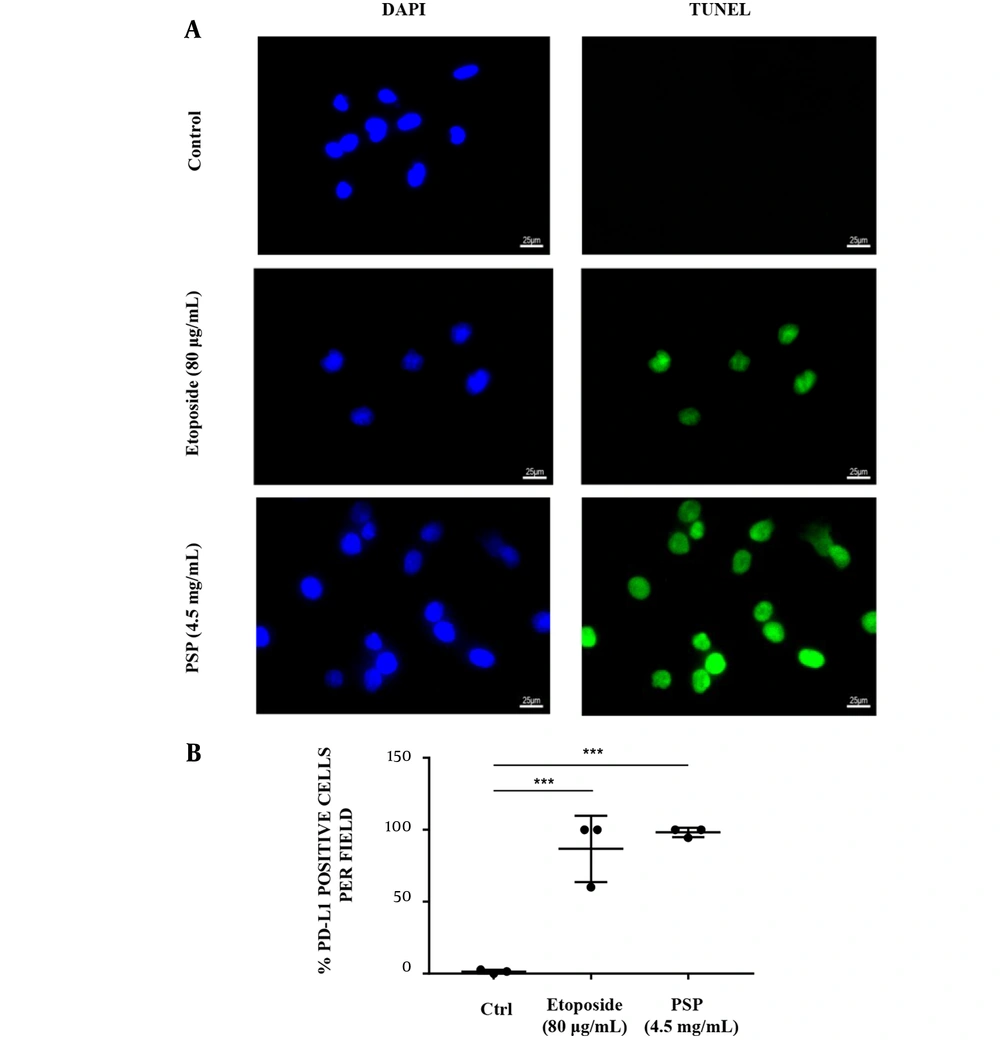
Pre-treated with PSP, the PD-L1 expression in HCT116 cells membrane was significantly down-regulated (Figure 4A and B), and HCT116 treated with PBMC was more responsive to the immunogenic cell death than untreated HCT116 and positive control. The TUNEL signal per cell revealed that pre-treated with PSP may trigger the immunogenic cell death (ICD) of CRC (Figure 5A and B).
The immunogenic cell death (ICD) effect of immune cells provoked by polysaccharide peptide pretreatment. A, HCT116 cells were seeded (3 × 104/well) on glass coverslips in medium and/or treated with Etoposide (20 μg/mL) or PSP (4.5 mg/mL, 48 hours). Activated peripheral blood mononuclear cells were co-cultured with HCT116 (PBMC: HCT116 = 5: 1) in the culture plate for 24 hours. The cells were detected with transferase-mediated nick-end labeling assay and imaged by fluorescent microscope. The experiments were repeated three times; B, The mean value of positive rates of TUNEL per field in each view with PSP or Etoposide treatment was compared to the untreated control. * P < 0.05, ** P < 0.01, *** P < 0.001, t-test.
5. Discussion
Polysaccharide peptide has been used in the treatment of cancer, hepatitis, hyperlipidemia, chronic bronchitis, and other diseases in clinical trials (32). Polysaccharide peptide has been shown to have low cytotoxicity (33), as well as synergistic and attenuating effects with chemotherapeutic agents (22). Polysaccharide peptide has previously been demonstrated to have an immunoregulatory effect by regulating the functions of multiple types of immune cells. Also, it has demonstrated anti-proliferative activity against cancer and controlled immune response (20, 34). Polysaccharide peptide anti-tumor effects are mainly tested using human leukemia HL60 cell lines (35), which can inhibit the proliferation of tumor cells by preventing the tumor cell cycle (20) and inducing apoptosis (36). Even though Roca-Lema et al. (37) and Knezevic et al. (38) showed that PSP had anti-tumor effect on CRC, there has been little research into the mechanism of PSP involvement in immune regulation.
Epidermal growth factor receptor activation triggers signaling pathways like RAS, PI3K, and STAT3, which contribute to cancer cells development and progression (39). Epidermal growth factor receptor inhibitors, like Gefitinib, are the most commonly used drugs in cancer treatment, but neoplasm drug resistance makes them ineffective. Macrophages, endothelial cells, and other non-malignant cell types express PD-L1, which allows T lymphocytes to specifically target and eliminate abnormal cells (10). Tumor cells that express PD-L1 can elude immune surveillance and even stimulate immune cells to defend themselves. Programmed cell death-ligand 1 expression was substantially correlated with EGFR (18). Polysaccharide peptide significantly inhibited the proliferation of HCT116 and HT29 in this study (Figure 1). Some researchers found that EGFR inhibitors decreased the PD-L1 expression in cancer cells (40). Our study consistently discovered that PSP suppressed the expression of EGFR and PD-L1, as validated by qRT-PCR, western blotting, and immunofluorescence. So, there might be a link between EGFR and PD-L1. This study evaluated the signaling pathways that might be involved in PSP’s ability to inhibit tumor cell growth. The level of EGFR site-specific phosphorylation and EGFR expression were decreased after PSP treatment. Polysaccharide peptide therapy reduced intracellular EGFR signaling cascade intermediates, such as EGFR, p-EGFR (Tyr1068), STAT3, p-STAT3 (Tyr705), and c-Jun expression and phosphorylation (Figure 3). The EGFR pathway’s downstream signaling molecules were regulated at varied levels. Curcumin has been shown to suppress p-STAT3 (Tyr705) and diminish the expression of PD-L1 in head and neck cancer (41).
This study had some limitations. First, the used PSP was bought from natural resource of Trametes versicolor and consistently demonstrated CRC cells anti-proliferative activity in vitro. It is necessary to create an index of PSP purity and anti-tumor activity to monitor this consistency. Second, anti-proliferative activity of PSP was tested only in two CRC cells. So, it will be more persuasive to test its anti-proliferative activity in more CRC cell lines and tumor xenograft animal model. This can enhance the chance of applying PSP as a safe prophylaxis and therapeutic agent. Third, our results showed that PSP could attenuate PD-L1 and EGFR related signaling to inhibit the CRC proliferation. More extensive experiments are needed to explore the molecule mechanism of EGFR involvement in PSP inhibition of CRC proliferation. Finally, our results showed that PSP inhibited the CRC cells proliferation via down-regulating expression of PD-L1 and EGFR. It is necessary to investigate other pathways that contribute to immunogenic cell death.
In summary, PSP suppresses CRC cell proliferation by inhibiting key signaling pathways involved in tumor growth and metastasis, indicating that it has a tremendous potential as a PD-L1 inhibitor and immunological supplement in the treatment of CRC. Purified PSP could also be manufactured and used as a safe preventative and treatment medication for colorectal and probably other malignancies with EGFR mutations. Also, PSP reduced PD-L1 expression, implying that it could be used as an immune supplement.
5.1. Conclusions
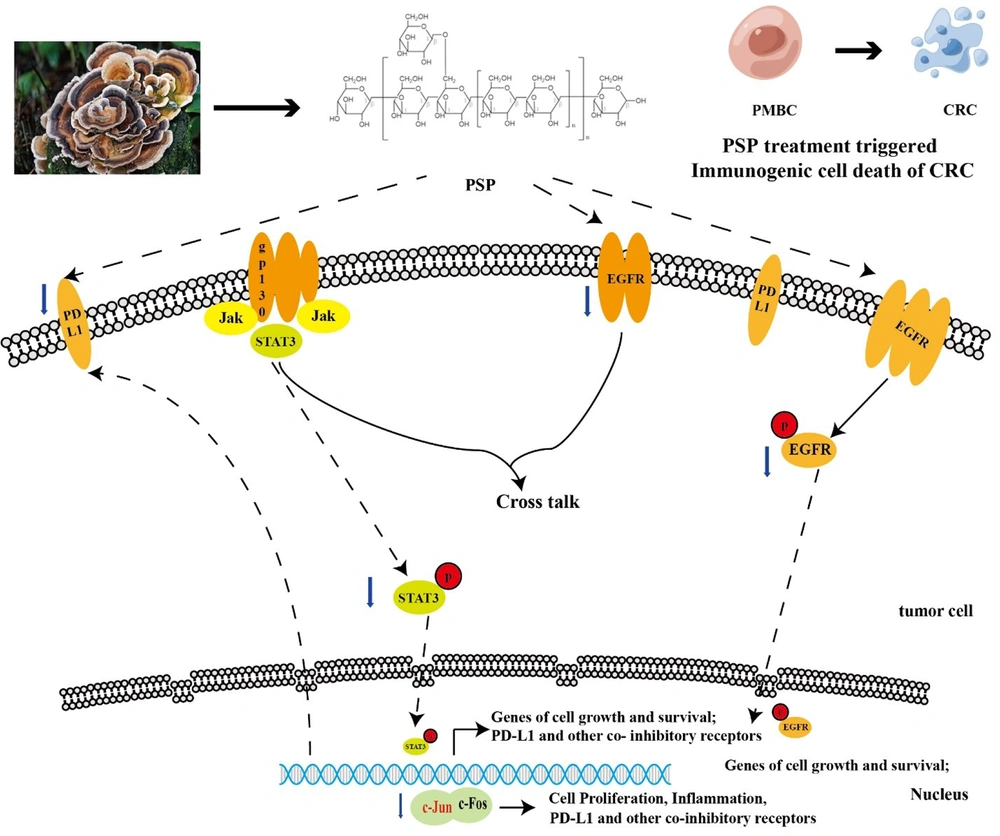
In this study, we proposed that PD-L1 might depend on EGFR activation. Then, when the expression of PD-L1 is down-regulated, immune cells recognize and annihilate CRC cells. PSP not only down-regulated EGFR expression but also promoted death in CRC cells via EGFR pathways (Figure 6).