1. Background
Changes in quality specifications of drug products can potentially influence therapeutic efficacy and even result in an unacceptable clinical drug safety profile. In this regard, semisolids possess many quality attributes that may affect the drug permeation through the skin and thus their therapeutic efficacy (1). Water content is recognized as one of the critical quality attributes of topical semisolid dosage forms because after applying topical dosage forms on the skin, their volatile components, including water evaporate (2). For example, in the case of emulsions, the partial volume of oil and water phases can affect the type of emulsion (W/O or O/W) (3), drug release and penetration (4), and thermodynamic activity of the drug (5). Some studies carried out on the liquid emulsions have shown that during the application of an emulsion, the volatile components evaporate and, therefore, phase inversion (6), flocculation (7), coalescence (7, 8), and change of evaporation rate (8) may occur. In addition, studies on emulsions have indicated that the type of emulsion (O/W or W/O) affects skin drug delivery significantly. Due to the evaporation of the volatile components of the emulsions, there is a possibility of resizing the droplets. Besides, some studies have shown that the skin penetration depends on the size of the droplets in the emulsion, so that in the same conditions, the smaller the size of droplets, the more the probability of skin absorption of drug molecules (9).
Creams are semisolid emulsions, and it is anticipated that after applying creams on the skin and subsequent water evaporation, their physical properties would change. As most O/W creams are applied and rubbed onto the skin as a thin film, a dynamic evaporating film generates. In this way, the relative concentrations of the volatile ingredients change. Subsequently, the dissolution and partitioning environment alters. Thus, the rapid evaporation may temporarily supersaturate the film, increasing the thermodynamic activity and drug permeation (10). It has been shown that in the case of hydrocortisone O/W creams containing a nonionic emulsifier, by increasing the amount of water in the cream to 60%, although the in-vitro release of hydrocortisone was delayed, the amount of release increased, possibly due to a change in the liquid crystalline microstructure of the cream (4). A study carried out on liquid emulsions composed of soya oil, polysorbate 80 and water have shown that during the application of the emulsion, the volatile components evaporate and phase inversion occurs (6). On the other hand, studies on the emulsions have indicated that the type of emulsion (O/W or W/O) affects the skin drug delivery significantly. For example, the skin penetration of hydrophilic drugs increases when they are in the continuous phase of an O/W emulsion (11).
Although creams' physical properties and water content are introduced as a critical quality attribute, which may affect the quality of formulation and its efficacy, there is no study on the effects of water content changes on the quality of creams after application over time. Therefore, in this investigation, the water content of different types of creams as a variable parameter has been studied, especially after application on the skin over time. Besides, the correlation between the water content of formulations and rate of water loss, droplet size, emulsion type, and occlusivity have been studied. Furthermore, an in-vitro model for studying the water content and other physicochemical properties of cream has been introduced and compared with cream applied on mouse skin.
2. Methods
2.1. Materials
Cetyl alcohol (> 96.0%), stearyl alcohol (> 96%), sodium dodecyl sulfate (SDS) (90%), and sodium tetraborate decahydrate (99.5 - 103.0%) were purchased from Merck (Germany). Ketamin hydrochloride (50 mg/mL) was supplied by RotexMedica (Germany), and xylazine (2%) was obtained from Alfasan Woerden (the Netherlands). Amaranth was provided by Fluka (Switzerland). Spermaceti and beeswax were purchased from Pishgaman Shimi (Iran), and white Vaseline was supplied by Rose Shimi (Iran).
2.2. Preparation of Creams
Five different formulations were prepared using the mixing method. The designed formulations were of both O/W and W/O types, each type with and without NaCl as a humectant (Table 1).
| Ingredients | Formulations | ||||
|---|---|---|---|---|---|
| A | B | C | D | E | |
| Cetostearyl alcohol | - | - | - | 7.8 | 7.8 |
| White Vaseline ® | - | - | - | 15 | 15 |
| Spermaceti | 5 | 7 | 7 | - | - |
| White bees wax | 5 | 7 | 7 | - | - |
| Liquid paraffin | 48 | 59 | 59 | 6 | 6 |
| Sodium borate | 0.5 | 0.5 | 0.5 | - | - |
| Sodium dodecyl sulfate | - | - | - | 1.3 | 1.3 |
| NaCl | - | - | 2 | - | 2 |
| Water | to 100 | to 100 | to 100 | to 100 | to 100 |
Formulations were prepared by separately heating the oil phase (70°C) and the aqueous phase (75°C). Afterward, the aqueous phase was added to the oil phase under continuous stirring (2000 rpm) at about 70°C for 10 min using WiseStir HS-D digital high-speed overhead stirrer. To obtain a semisolid emulsion, the resulting emulsion was allowed to cool down gradually to room temperature under constant stirring (300 rpm). These creams were stable for at least 14 days in well-closed plastic jars at room temperature.
2.3. In-Vitro and In-Vivo Creams Application and Water Loss Models
To investigate changes of creams upon application, three methods were employed here: (1) in-vitro (applying on aluminum sheets); (2) in-vivo (applying on mouse skin); and (3) infinite dose methods (using thermogravimetric analysis, TGA), as follows.
2.4. In-Vitro Aluminum Sheet Model
In the in-vitro method, 5 mg/cm2 of the creams were uniformly spread on aluminum sheets, and sheets were then placed in the oven for 1, 2, and 4 hours at 32°C. The applied creams were then harvested, and their physicochemical properties, including the occlusivity, water loss, type, and size, were studied, as described later in detail.
2.5. Mouse Skin Model
In the in-vivo method, the creams were uniformly applied on the shaved abdominal skin adult male albino mice (25 - 35 g) anesthetized using ketamine and xylazine. The in-vivo experiments complied with the defined ethical codes in Shahid Beheshti University of Medical Sciences. A warm electric blanket was used to keep the normal body temperature of mice. Five mg/cm2 of cream was uniformly applied to the skin using a glass rod and left for 1, 2, and 4 h. Thereupon, the applied cream was harvested, and its physicochemical properties, including type and droplet size, were studied, as described later. Droplet size and emulsion type of in-vitro and in-vivo methods were also compared for validation.
2.6. TGA- Infinite Dose Model
To determine the continuous water evaporation trend, an infinite dose of cream (25 mg/cm2) was directly placed into the TGA pan of the Shimadzu TGA-50 instrument (Japan). The system was kept at 32°C (skin temperature) for 4 h, and the changes in sample weight, which show the amount of water loss, were recorded.
2.7. Water Evaporation Rate
In order to evaluate the water release (evaporation) rate in the infinite dose method and the in-vitro aluminum sheet model, the TGA-50 instrument was used. In the case of in-vitro aluminum sheet model, the applied creams on aluminum sheets (5 mg/cm2) were harvested after 1, 2, and 4 h being kept at 32°C in the oven. The harvested creams in different time intervals were placed into TGA pans separately, and the remained water in the creams was estimated using weight loss after heating at 5°C/min over the temperature range of 25 - 150°C. In the infinite dose model, 25 mg/cm2 of creams was directly placed into the TGA pan. The temperature of the TGA instrument was kept at 32°C for 4 h and according to the weight loss of samples, the remained water in the creams was estimated. To estimate water evaporation rate in both in-vitro and infinite dose methods, the percentage of water loss was plotted against time post-application. The slope of such a diagram indicates the rate of water loss. Each experiment was performed in triplicate.
2.8. Physicochemical Characterizations
2.8.1. Determination of Creams Type (O/W vs. W/O)
The dye solubility test and electrical resistance measurement were performed to determine the type of creams. The study for each cream was repeated three times.
"Dye solubility test" was performed as a rapid and easy test to identify the emulsion type (5). In this test, Amarant was used as a water-soluble dye to determine emulsion type here.
The same-colored systems were used for the microscopic observation using an optical microscope (MICROS AUSTRIA 05063).
The electrical resistance measurement method was also used for the determination of the cream type. In this method, the electrical resistance of the cream is measured by an ohmmeter. To measure the electrical resistance of creams in the present study, the probes of Digital Multimeter DT9208A (AKB, China) were put into a vial containing the cream. As the highest value for resistance in the device was 200 MΩ, a 200 MΩ on the screen was interpreted as the W/O cream.
2.8.2 Droplet Size Measurement
The droplets of the dispersed phase per square in the Neubauer chamber (HBG, Germany) were measured with a gridded ocular lens of optical microscope (MICROS 05063, Austria) and 400X total magnification. For each cream, the measurement was repeated three times, and each time 5 small squares were randomly selected and analyzed for droplet sizes.
2.8.3. Determination of Occlusivity
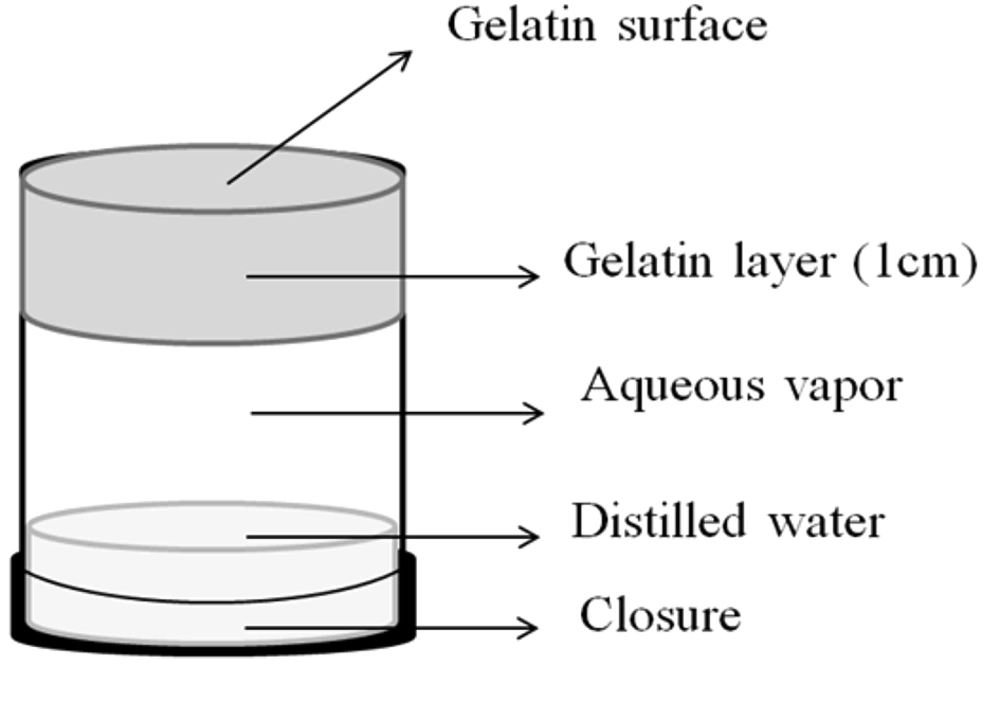
Gelatin cells were used for the determination of occlusivity of creams after application. This method measures the evaporation of water from a gelatin layer and the ability of vehicles to prevent water evaporation. This hydrated gelatin membrane is an indicator of cream occlusivity (12). Gelatin cells were prepared using the method developed by Handjani-Vila et al. (13) with a slight modification. To prepare cells, hot gelatin aqueous solution (12% w/w) was prepared and spread on a petri dish in 1 cm thickness. Plastic hollow cylinders (with 5 cm in height and 2.5 cm in diameter) were immersed in the gelatin solution, and the system was placed in the refrigerator for 2 h to be sealed on one side (top) by gelatin. The gelatin-sealed plastic cylinder was then removed and used.
To ensure 100% relative humidity inside the cell, 5 mL water was poured into the cell, and the bottom of the cylinder was closed with a cap (Figure 1). Gelatin cells were placed in a desiccator at room temperature with their gelatin ends faced upwards (Figure 1). Five mg/cm2 creams were spread on the gelatin surface of sample cells, and no cream was applied on the gelatin surface of blank cells. To assess the occlusivity of the creams, changes in weight of the sample and blank cells were evaluated over 0, 25, 60, 120, and 240 min after application of creams. A cream was considered occlusive if the water loss of sample cells were significantly less than the sum of water loss of blank cells (without cream) and the water loss from the cream alone (obtained from the TGA data of in-vitro aluminum sheets model). P-values less than 0.05 were considered as significant weight differences. Each experiment was run in triplicate.
2.9. Statistical Analysis
Statistical analysis of the results was performed by unpaired t-test using the GraphPad Prism version 6.07 software. P-values less than 0.05 were considered as a significant difference.
3. Results and Discussion
3.1. Determination of Cream Type
Table 2 shows the types of different cream formulations at time zero (immediately after preparation), 1, 2, and 4 h after application on aluminum sheets, determined by dye solubility test and electrical resistance measurements. The results were the same for both methods. In the case of electrical resistance measurement, W/O cream showed 200 MΩ electrical resistance (the upper limit of measurement by the device), while O/W creams showed resistance of 0.90 - 3.14 MΩ. In the dye solubility test, the macroscopic view of W/O creams was gray, and there were pink droplets (water) dispersed in a colorless background (oil) in their microscopic view. On the contrary, O/W creams were pink in the macroscopic view, and colorless droplets (oil) were observed on a pink background (water) under the optical microscope.
| Formulation | NaCl | Time After Application (h) | |||
|---|---|---|---|---|---|
| Time Zero | 1 | 2 | 4 | ||
| A | - | O/W | W/O | W/O | W/O |
| B | - | W/O | W/O | W/O | W/O |
| C | + | W/O | W/O | W/O | W/O |
| D | - | O/W | W/O | W/O | W/O |
| E | + | O/W | W/O | W/O | W/O |
As shown in Table 2, the O/W creams (formulations A, D, and E) inverted to W/O just one hour after application on aluminum sheets. The presence of NaCl as a humectant in formulation E did not prevent such phase inversion. In contrast, the phases of W/O creams were stable after applying and did not invert. The phase inversion could be related to the evaporation of water from the applied cream and consequently reduction of the aqueous phase (1, 5, and 6). Friberg and Langlois have demonstrated that the phase inversion occurred 1 h after applying liquid emulsions (8). Since the emulsion type is affected by the volume ratio of aqueous and oily phases (3), this phase inversion can be attributed to the water evaporation of creams as semisolid emulsions. Phase inversion, as seen in the case of liquid emulsions (11), is likely to affect the pattern of drug release from an emulsion. In addition, the consumer feeling of W/O products is different from O/W products. W/O products cannot be washed with water easily and are not suitable for moist wounds (14). Therefore, it is important to know the fate of emulsions after application.
As mentioned earlier, since phase inversion in W/O creams did not occur, it seems that the phase inversion of the O/W cream is due to the reduction of the continuous phase volume following water loss over time. Accordingly, it is expected that the drug release or other properties of creams would be more stable and predictable in W/O creams than O/W types.
Although the use of 1 - 5% humectant can be reasonable in keeping humidity in creams and lotions (15), comparing formulations D and E shows that 2% NaCl (in formulation E) is not effective in preventing the phase inversion after 1 hour. Regarding the role of humectant in skin formulations, the inability to prevent water loss in this case probably is related to the low concentration of humectant in the formulation. It should be noted that increasing the concentration of NaCl led to phase separation in formulations C and E.
Formulations A, B, and C were also applied to the mouse skin (in-vivo method). The results were similar to that of the in-vitro aluminum sheet model. The O/W cream (formulation A) inverted to W/O just after 1 h, while there was no change in W/O creams (formulations B and C) after application (Table 3). Effect of humectant in preventing phase inversion of W/O creams is not perceptible here; since the investigated cream containing NaCl (formulation C) is a W/O one, and decreasing internal phase is not effective on the type of creams.
| Formulation | NaCl | Time After Application (h) | |||
|---|---|---|---|---|---|
| Time Zero | 1 | 2 | 4 | ||
| A | - | O/W | W/O | W/O | W/O |
| B | - | W/O | W/O | W/O | W/O |
| C | + | W/O | W/O | W/O | W/O |
Because the results of the in-vivo method agree with the in-vitro aluminum sheet model, it could be concluded that the in-vitro aluminum sheet model is almost reliable for such kind of experiment, and the aluminum sheets can be used as a good model for studying creams phase inversion after topical application.
3.2. Droplet Size Measurement
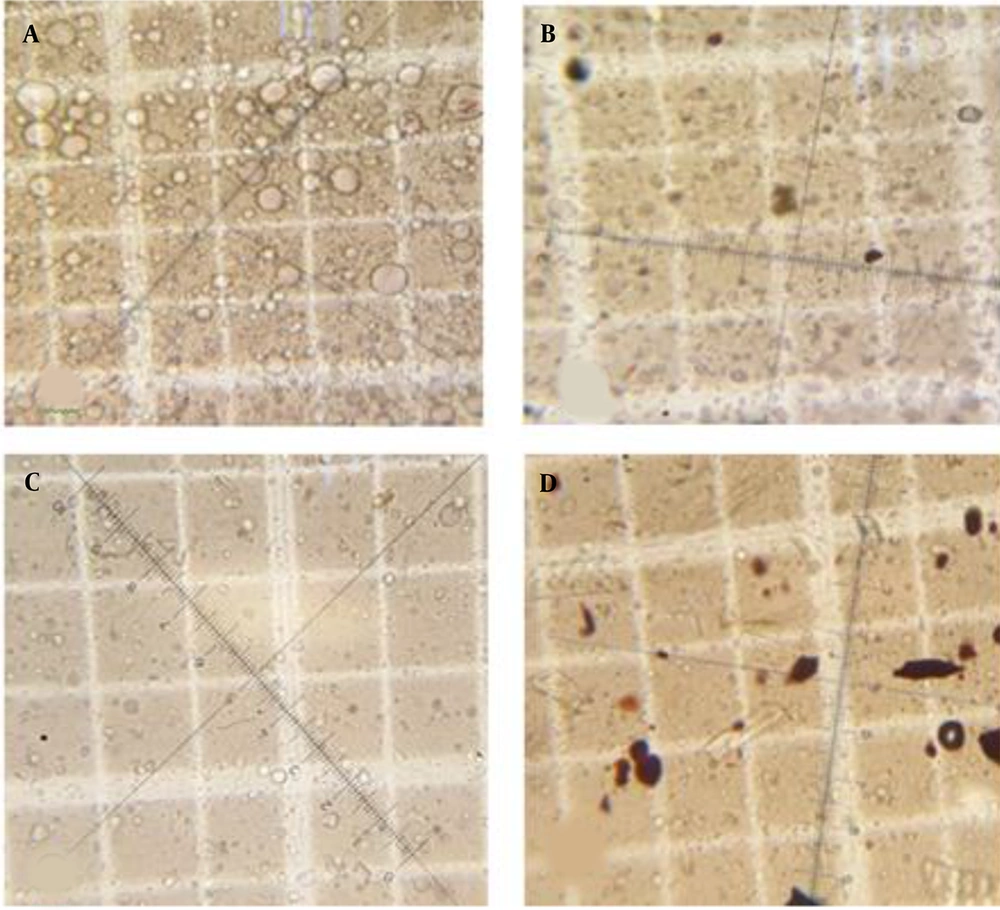
The initial droplet size at zero time, 1, 2, and 4 hours after application of the formulations to the skin for formulations A - C are summarized in Table 4. The microscopic pictures of formulation A are demonstrated in Figure 2. The number and the mean diameter of the droplets reduced in all three formulations over time. This reduction in the size of droplets should be due to the evaporation of water from the cream after the application and the reduction in the water content of the formulations (7).
| Formulation | Type | NaCl | Time After Application (h) c | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Time Zero | 1 | 2 | 4 | ||||||
| Size | Size | PI | Size | PI | Size | PI | |||
| A | O/W | - | 17.04 ± 0.76 | 3.83 ± 0.45 A | + | 3.58 ± 0.52 B | - | 0.83 ± 1.44 | - |
| B | W/O | - | 7.70 ± 0.48 | * | - | * | - | * | - |
| C | W/O | + | 9.99 ± 1.05 | 6.41 ± 0.27 | - | 2.41 ± 0.91 C | - | 3.86 ± 0.52 D | - |
a Values are expressed as mean ± SD.
b The size of droplets was less than 1 μm.
c P-value between capital letters, A and B > 0.05; and between C and D > 0.05.
After applying the cream, the presence of humectant in formulation C considerably prevented the reduction in size of the droplets compared to its similar formulation (formulation B). This phenomenon confirms the above-mentioned discussion regarding the effects of water evaporation on droplet size. It also suggests that humectants can affect the process of emulsion changes upon application.
To compare the efficiency of in-vitro aluminum sheet model with in-vivo method in the measurement of droplet size, the changes in the droplet size of formulation A was investigated by both methods over time (Table 5). As expected, in both methods, the average size of droplets gradually decreased after application over time. Statistical analysis showed that the mean diameter of droplets in the cream applied to the aluminum sheets was not significantly different from that of the cream used to the skin (P > 0.05).
| Method | Time After Application (h) | |||
|---|---|---|---|---|
| Time Zero | 1 | 2 | 4 | |
| Aluminum | 17.04 ± 0.76 | 3.44 ± 0.09 | 2.75 ± 0.16 | 0.88 ± 1.5 |
| Skin | 17.04 ± 0.76 | 3.83 ± 0.45 | 3.58 ± 0.52 | 0.83 ± 1.44 |
| P-value | - | 0.2169 | 0.0574 | 0.9688 |
a Values are expressed as mean ± SD.
According to these results and the results obtained from the determination of cream type, it can be concluded that studies based on the in-vitro aluminum sheet model are reliable for the physical evaluation of cream and can almost represent the conditions of the skin. The importance of using this model is the lack of ethical problems because there is no need to use animals in such in-vitro models.
3.3. Determination of Occlusivity
The changes in the occlusivity of creams after application on the gelatin cell are presented in Table 6. All formulations were occlusive at the end of the study without any exceptions. Formulation A (O/W) that shows no occlusivity at time zero became occlusive after about 25 min; this is probably due to the inversion of the phases and change to the W/O cream, as discussed earlier. Formulations B and C (W/O) also became occlusive after about 25 min; this observation can be related to the evaporation of water droplets and the formation of oily films. The occlusivity of formulations D and E (O/W) increased over time as well. A comparison between the occlusivity changes of formulation B with formulation C (containing humectant) shows that the humectant had no effect on the occlusivity over time. However, the occlusivity of O/W cream (formulation D) was affected by humectant, so formulation E (containing humectant) became occlusive much sooner than formulation D. Based on the results, the humectant seems more effective in occlusivity when it is in the continuous phase of creams. Choudhury et al. have also shown that increasing the concentration of humectant in emulsion formulation has changed the occlusivity (16).
| Formulation | Type of Cream | Time Ranges After Application on the Gelatin Cell (min) a | |||
|---|---|---|---|---|---|
| 0 - 25 | 25 - 60 | 60 - 120 | 120 - 240 | ||
| A | O/W | - | + | + | + |
| B | W/O | - | + | + | + |
| C | W/O+NaCl | - | + | + | + |
| D | O/W | - | - | - | + |
| E | O/W+NaCl | - | + | + | + |
a -, non occlusive; +, occlusive.
3.4. Water loss
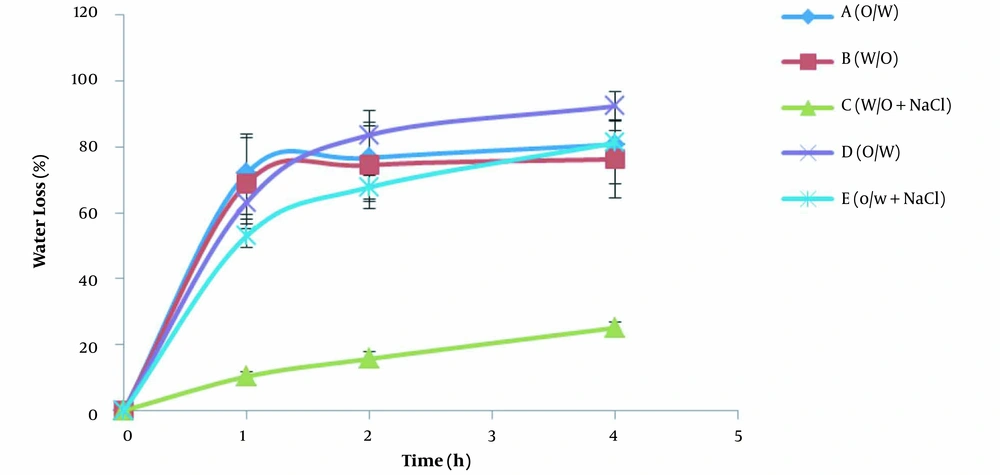
3.4.1. Water Loss in the In-Vitro Aluminum Sheet Model
The water contents of creams obtained by TGA after the application on aluminum sheets (spreading 5 mg/cm2 of the cream on a large surface) and the storage at 32°C in an oven are presented in Table 7. The results showed that water contents of all formulations reduced up to 70 - 90% one hour after application. Two hours after application of the creams, regardless of the type of cream, the formulation, and the presence of humectant, the water content reached about 5% of the initial, and after four hours, there was negligible water left in all creams (less than 4%). Considering the importance of the water amount in topical drug delivery, it is necessary to offer an approach to reduce the amount of evaporation. Covering the surface of the skin after cream application and the use of enough humectant might be helpful.
| Formulation | Formulation Type | Percentage of Remained Water Content (h) | |||
|---|---|---|---|---|---|
| Time Zero | 1 | 2 | 4 | ||
| A | O/W | 31.17 ± 3.12 | 9.01 ± 1.39 | 1.73 ± 0.54 | 0.49 ± 0.06 |
| B | W/O | 22.04 ± 1.32 | 3.79 ± 2.62 | 1.25 ± 0.75 | 0.43 ± 0.23 |
| C | W/O + NaCl | 21.89 ± 0.54 | 4.97 ± 3.25 | 0.72 ± 0.43 | 0.56 ± 0.18 |
| P-value between B and C | - | 0.65 | 0.35 | 0.48 | |
| D | O/W | 58.99 ± 2.31 | 4.08 ± 0.27 | 3.56 ± 0.46 | 3.52 ± 0.1 |
| E | O/W + NaCl | 58.36 ± 3.38 | 6.15 ± 0.64 | 3.95 ± 0.20 | 3.70 ± 0.13 |
| P-values between D and E | - | 0.006 | 0.25 | 0.13 | |
a Values are expressed as mean ± SD.
W/O formulations B and C, regardless of humectant presence, had no significant difference in water content at any time after application (P > 0.05). Comparison of O/W formulations D and E with and without humectant also demonstrated that O/W formulations were so close in water content. However, formulation E had a higher water content in the first hour after application (P < 0.05). Since both formulations D and E were converted to W/O creams in less than one hour after application due to the phase inversion, they showed a similar trend to the W/O formulations used in this study (formulations B and C). Based on the mentioned results, the presence of NaCl did not affect the water evaporation in the in-vitro aluminum sheet model. The reason for this observation can be attributed to the low NaCl content (2%), which cannot effectively reserve water in creams applied on the aluminum sheet, where the evaporation rate is high. Note that using more NaCl was not possible due to the instability of formulations in a higher amount of NaCl. As will be seen later in the next section, even this low NaCl content can preserve water when the thickness of the applied cream is increased.
3.4.2. Water Loss in the Infinite Dose Method
The results of the remained water content of creams applied into the TGA pan in the amount of 25 mg/cm2 for 4 h at 32°C are provided in Table 8. The results indicated that the water content reduced over time in all formulations so that only about 4 - 16% of water remained in creams after 4 hours. These results are in agreement with the in-vitro aluminum sheet model.
| Formulation | Formulation Type | Percentage of Remained Water Content (h) | |||
|---|---|---|---|---|---|
| Time Zero | 1 | 2 | 4 | ||
| A | O/W | 31.17 ± 3.12 | 8.73 ± 3.73 | 7.28 ± 3.05 | 6.00 ± 2.33 |
| B | W/O | 22.04 ± 1.32 | 6.84 ± 3.04 | 5.63 ± 2.89 | 5.23 ± 2.61 |
| C | W/O + NaCl | 21.89 ± 0.54 | 19.63 ± 0.32 | 18.45 ± 0.47 | 16.39 ± 0.40 |
| P-value between B and C | - | 0.0019 | 0.0016 | 0.0019 | |
| D | O/W | 58.99 ± 2.31 | 21.72 ± 2.14 | 9.67 ± 4.42 | 4.28 ± 2.61 |
| E | O/W + NaCl | 58.36 ± 3.38 | 27.43 ± 2.11 | 18.82 ± 2.11 | 10.96 ± 2.13 |
| P-values between D and E | - | 0.0302 | 0.013 | 0.0264 | |
a Values are expressed as mean ± SD.
Contrary to the in-vitro model, in the infinite dose method, the presence of 2% NaCl was effective on water evaporation of W/O and O/W creams. The water evaporation in formulations containing humectant i.e. formulations C (W/O) and E (O/W), overtime was significantly less than that of in formulations B (W/O) and D (O/W) without humectant, respectively (P < 0.05). This difference in the results obtained from the in-vitro aluminum sheet model and infinite dose method can be attributed to the different thicknesses of the cream placed in the pan and applied to the aluminum sheets. In a comparative point of view, in the infinite dose conditions, more amount (25 mg/cm2) of cream was placed in a smaller surface i.e. TGA pan, whereas in the in-vitro aluminum sheet model, less amount (5 mg/cm2) of cream was applied on a larger surface i.e. aluminum sheet, indicating the more thickness of cream in the infinite dose method. Given this point, it seems that there was more water in the infinite dose method to compensate for the lost moisture from the surface, compared with the aluminum sheet model. Consequently, there was probably enough water in the pan so that the humectant could hold water molecules more efficiently over time. Therefore, to reduce the amount of water evaporation using humectant, the increased thickness of applied cream on the skin might be useful.
3.4.3. Water Evaporation Rate
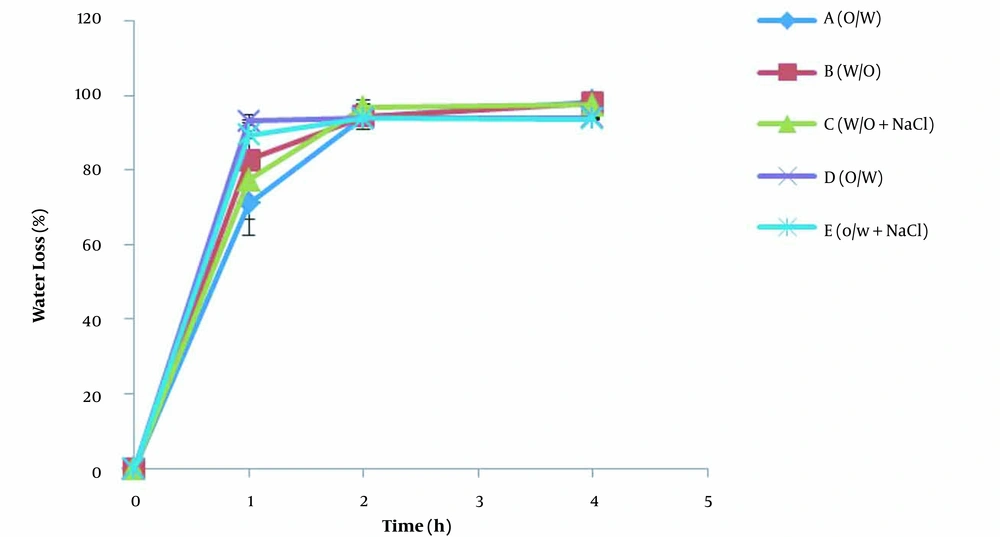
The changes in the percentage of water loss of creams after application on the aluminum sheets (in-vitro method) and in the TGA pan (infinite dose method) over time are shown in Figures 3 and 4, respectively. As mentioned earlier, the slopes of the diagrams indicate the water evaporation rate. There are two distinct areas of water loss in the diagrams of both methods. At first, the water molecules are released from the creams with a high evaporation rate that ends somehow by a plateau. These observations can be attributed to the reduction in the water content of creams over time and subsequently to the decrease in the concentration gradient of water molecules. Of course, there were some differences between the patterns of water evaporation in the in-vitro aluminum sheet model and the infinite dose method. In the in-vitro aluminum sheet model (Figure 3), the water evaporation rate was high at first for all formulations and then reduced a lot. In the case of the infinite dose method (Figure 4), although the evaporation rate of water from all creams decreased over time, the rate of water loss in creams containing humectant (formulations C and E) was less than that of the same creams without humectant (formulations B and D). In addition, all creams in the infinite dose method reached the maximum water loss slower than the creams in the in-vitro aluminum sheet model. As discussed in the water loss section, these differences could be due to applied creams’ different amounts and thicknesses in the in-vitro aluminum sheet model and infinite dose method. In order to interpret, it is helpful to assume that the applied cream on the surface is composed of consecutive layers. The water molecules present at the superficial layers of creams evaporate and form water vapor. Therefore, a depletion zone is created inside the creams. Some water molecules from the lower layers of the cream diffuse, fill the depletion zone, and then evaporate. In the infinite dose method, where the amount and thickness of applied cream are high, more water molecules are available to fill the receding depletion zone. Furthermore, the humectant/water ratio increases in the depletion zone. The humectant molecules in such conditions would be able to reduce the escaping tendency of water molecules more efficiently and consequently reduce the evaporation rate.
4. Conclusion
The present study evaluated the changes in cream properties following topical application and their influence on the product efficiency. The results revealed that some important characteristics of creams changed after application. These changes included the inversion of O/W cream phases, alteration in the occlusivity of cream, and decreasing the water content, rate of water evaporation, and size of the droplets. Furthermore, the results demonstrated that the in-vitro aluminum sheet model imitated the real skin condition to an acceptable extent. The results also showed that increasing the thickness of the applied cream on the skin decreased the intensity of the changes. Therefore, where the change is critical, the amount of the cream should be increased. Also, it might be necessary to increase the recommended amount of applied to amounts higher than what is currently recommended, i.e. 5 mg/cm2. The present results clearly show that creams do not stay unchanged after application to the skin and suggest that formulators should be aware of such possible changes and, therefore, required precautions should be taken in advance.