1. Background
The spread of SARS-COV-2 has caused extensive changes in cognition and pathophysiology of viral diseases. Studies have shown that Coronavirus disease 2019 (COVID-19) is associated with different clinical stages and patients experience different symptoms at each stage (1, 2). According to a report released by the World Health Organization (WHO) in June 2021, more than 175 million people worldwide were infected with COVID-19 and 3,793,230 people died. At present, Iran ranks first among the various countries of the Eastern Mediterranean Region with 3,020,522 infected and 81,911deaths (2). More than a year after the pandemic began and widespread vaccination, COVID-19 is still a deadly disease. On the other hand, the emergence of new variants has added to the concerns of governments. Therefore, reviewing and managing local and national care and treatment protocols in each period can play an important role in integrating the approach presented in medical centers to reduce the costs of treatment, morbidity, and mortality of patients. In the present study, a summary of updated care guidelines was provided for hospitalized patients with COVID-19 at various levels, under the supervision of the National Tuberculosis and Lung Diseases Research Institute of Masih Daneshvari Hospital in Tehran, Iran.
This guideline has been compiled following the World Health Organization guidelines, the latest authoritative articles, and under the direct supervision of a team of physicians specializing in various fields of COVID-19 control. There is no conflict of interest for any of the members participating in this study.
2. Methods
After the COVID-19 pandemic, Masih Daneshvari Hospital in Tehran, Iran was introduced as one of the official COVID-19 case referral centers. In May 2020, the first management protocol for patients with COVID-19 admitted to the hospital was published by the team of specialists of this center. Due to the need to use the updated comprehensive management and treatment instructions, the present protocol is developed by a team of internal medicine specialists, pulmonologists, infectious disease specialists, clinical pharmacists, virologists, intensivists, and pathologists at Masih Daneshvari Hospital in Tehran, Iran in accordance with National Institutes of Health (NIH) and World Health Organization (WHO) and the latest authoritative articles.
3. Results and Discussion
3.1. Definitive Monitoring and Diagnosis
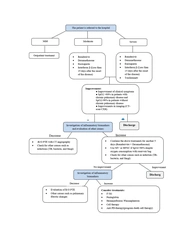
Diagnosis of COVID-19 is accomplished by assessing the patient's clinical symptoms, CT-scan and/or RT-PCR results (3).
3.1.1. CT-Scan
In general, the most common findings that can be seen on a CT-scan of patients with COVID-19 (with varying severity) include the following (4, 5):
- Ground-glass opacities (GGO): A CT-scan of these people found a white hazy opacities area in the lung tissue called "ground-glass opacities" that could be caused by thickening of the lungs or the overlapping part of the air sacs in the lungs.
- Crazy paving appearance: Ground-glass opacities with interlobular and interlobular septal thickening.
- Consolidations: Replacement of air inside the alveoli with exudative secretions.
- Interlobular septa: Thin linear opacities that were seen between pulmonary lobules.
- Halo Sign: The halo sign is around zone of ground-glass opacities that are seen around lung nodules at computed tomography (CT).
- Reversed Halo sign (atoll sign): A focal ground-glass attenuation surrounded by more compressed consolidation of crescentic shape.
Peripheral stripe-like opacity may also be seen if a few days have passed since the onset of symptoms. Findings are generally more common in the peripheral parts of the lungs (6).
3.1.2. Molecular Tests
Virology tests are performed by reverse transcriptase chain reaction (RT-PCR) (7). Sampling is performed using the necessary personal protective equipment with the help of nasopharyngeal or oropharyngeal swabs (8). Since the viral load in the upper respiratory tract samples is high in the first week of infection, preparing the mentioned samples using sterile swabs can be very effective in diagnosing the infection (9). According to the WHO recommendation, a positive molecular detection test for at least two different targets in the virus genome, one of which is specific for the virus, is sufficient to detect the COVID-19 virus in regions where the virus is not circulating (10). Also, the positive result of the molecular detection test for a typical target sequence between beta-coronaviruses and the SARS-COV-2 virus, which is confirmed by the sequencing test, is acceptable (11).
3.2. Classification of COVID-19 by Severity
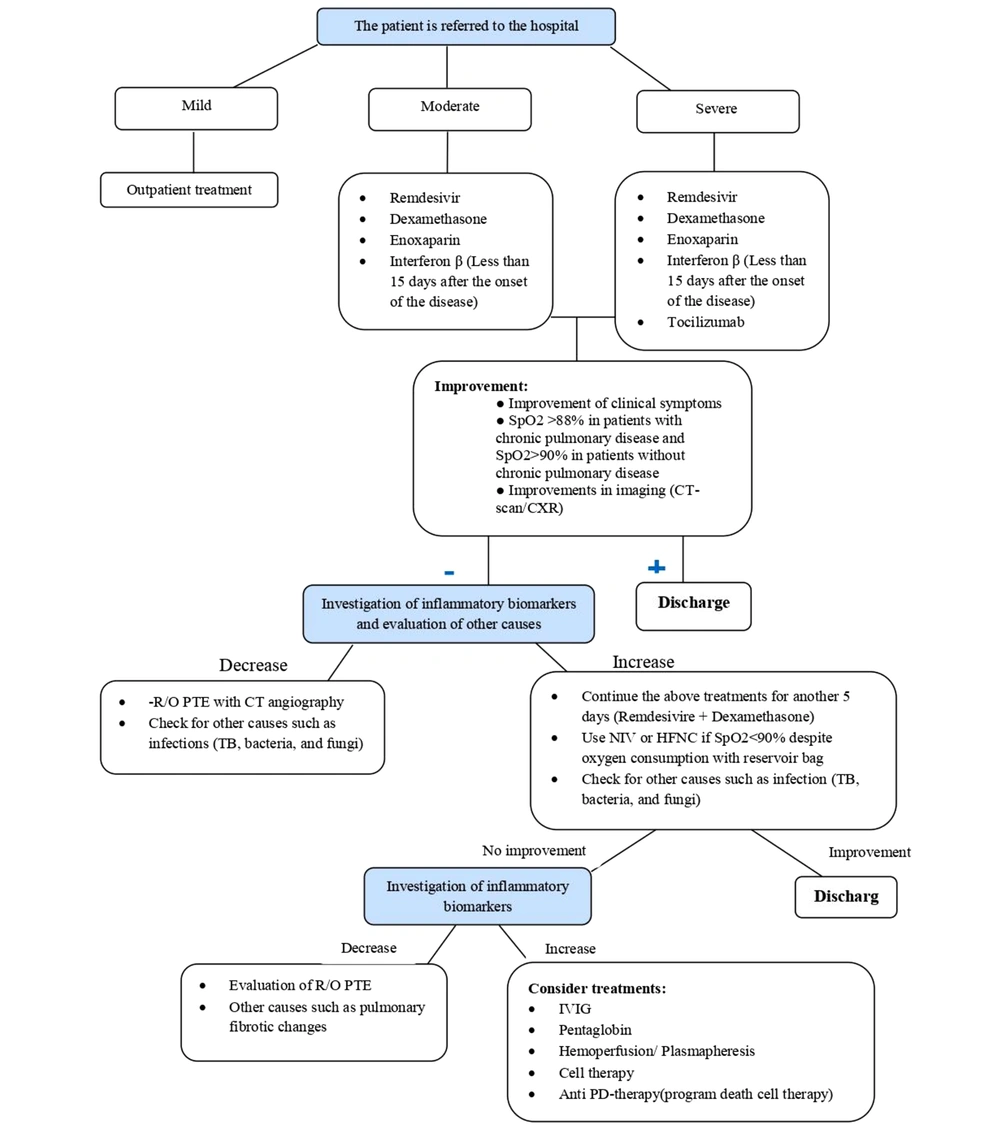
After the definitive diagnosis of COVID-19 and to perform effective treatment-care methods, it is necessary to determine the severity of the disease in each patient. Therefore, patients' conditions are divided into 4 categories based on the severity of the disease and clinical and laboratory manifestations: (1) mild, (2) moderate, (3) severe, and (4) critical (12). Patients in the mild group usually present to medical centers with the initial symptoms of the disease. These patients have no signs of disease progression and are receiving care and supportive (symptomatic treatment) treatments, and home quarantine is on the agenda to prevent the transmission of the disease to other people (13). Moderate and severe patients have indications for hospitalization in the ward and the necessary treatments are performed according to the patient's condition (14). Evidence of multi-organ dysfunction is also seen in some severe patients (15). These patients usually achieve SpO2 > 90% with the help of conventional oxygen. Details of each group are summarized in Table 1.
| Severity | Clinical symptoms | Drugs and procedure |
|---|---|---|
| Mild | SpO2 ≥ 94% on room air; Without hypoxia | Home quarantine; Social distance observation; Monitoring of body temperature and SpO2; Keep the body hydrated; Patient management through telemedicine or telephone visits (16) |
| Moderate | SpO2 = 90 - 93% on room air or 24 < respiratory rate < 30 breaths/min (17) Or Pulmonary infiltration < 50% (18) | Oxygen therapy until SpO2 levels improve; Dexamethasone: 8 mg/IV/daily for 5 to 10 days or until discharge (19); Enoxaparin: 40 mg/SC/daily (20); Ramdesivir: 200 mg/IV for the first day, 100 mg/IV for the next 4 days (21); and in severe cases (CRP ≥ 75 mg/L (22), high levels of IL-6 (23, 24), reduction of platelet and eosinophil (25): Tocilizumab: as a single dose of 400 mg drug diluted in 100 mL normal saline via IV infusion within one hour (22, 26) (*If the patient does not respond to these treatments, the patient was admitted to the intensive care unit and other treatments such as IVIG, Pentaglobin, hemoperfusion, plasmapheresis, cell therapy are used). |
| Severe | SpO2 < 90% on room air Or Respiratory rate > 30 breaths/min (27) or Pulmonary infiltration > 50% (26) | |
| Critical | SpO2 < 90% on room air; Requires oxygen via HFNC/NIV/mechanical ventilation (22) or pulmonary infiltration > 50% (28) or with hemodynamic disorders/multi-organ failure/coagulation disorders (23, 24); Usually high levels of IL-6 (29, 30). |
Abbreviations: IV, intravenous; SC, subcutaneous; HFNC, high-flow nasal cannula; NIV, non-invasive ventilation; CRP, C-reactive protein; IL-6, interleukin 6; IVIG, intravenous immune globulin.
3.3. Treatment of Patients with COVID-19 at Different Levels of the Disease
Generally, patients who do not have oxygen saturation disorders (SpO2 > 94%) and have mild symptoms of COVID-19 receive outpatient supportive and symptomatic treatment. Moderate and severe patients will be hospitalized. After hospitalization, oxygen therapy, and appropriate treatment line is used based on each patient's clinical condition, including antiviral and corticosteroid therapy, anticoagulant therapy, IL-6 inhibitors, and other required therapies.
3.3.1. Oxygen Therapy
The goal of oxygenation in hospitalized patients with COVID-19 is to achieve SpO2 > 90% in severe and SpO2 ≥ 94% in moderate cases. However, the percentage of optimal oxygen saturation for each person is affected by different parameters and has different values. Usually, in patients with COVID-19 associated with acute hypoxemic respiratory failure, conventional oxygen alone does not meet the needs of patients. Therefore, other methods such as high flow nasal cannula (HFNC), Noninvasive positive-pressure ventilation (NIPPV), invasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO) can be used individualized and according to the severity of the disease (31).
The nasal cannula is used for oxygenation in patients with COVID-19 with mild hypoxia. A maximum flow of 6 liters per minute can be provided to patients through the nasal cannula. Patients with moderate hypoxia also use a simple mask that provides an oxygen flow of 6 to 10 liters per minute. In severe hypoxia, a non-rebreather mask with an oxygen flow of 10 to 15 liters is used. If no significant increase in patient oxygen is observed with this method, NIV or HFNC is used (32).
3.3.2. Antiviral Therapy
Remdesivir is used as an FDA-approved antiviral drug to treat COVID-19 at 200 mg / IV for the first day, 100 mg/IV for the next 4 days. It binds to viral RNA-dependent RNA polymerase and prevents virus replication through premature termination of RNA transcription. The use of remdesivir is recommended for moderate to severe hospitalized patients who need supplemental oxygen (21). The use of this drug requires liver function tests (liver enzymes and prothrombin time). In cases where the level of alanine transaminase (ALT) is ten times higher than normal or there are signs of hepatitis, the drug should be stopped (33). The patient's renal function should also be evaluated before and while using remdesivir. This drug is not recommended in patients with severe renal impairment with GFR < 30 mL/min as well as advanced liver failure (34).
3.3.3. Corticosteroids
Patients with moderate to severe COVID-19 are usually associated with a systemic inflammatory reaction that can lead to lung damage and dysfunction of other organs. The use of corticosteroids can effectively prevent or control this condition with its anti-inflammatory effect. Studies have shown that the use of glucocorticosteroids such as dexamethasone 8 mg/ IV/daily for 5 days to 10 days or until discharge (equivalent to 200 mg of hydrocortisone or 40 mg of methylprednisolone or 50 mg of oral prednisone) can reduce the ventilation days and mortality (19). In the absence of clinical improvement, increased need for oxygen and SpO2 < 90% despite receiving supplemental oxygen and pulmonary involvement of more than 50%, individualized in each patient depending on the severity of the disease, methylprednisolone 250 - 500 mg/IV/daily is used for another 3 days. If dexamethasone is not available, alternative corticosteroids such as prednisone, methylprednisolone, and hydrocortisone can be used in doses equivalent to dexamethasone (20).
In general, previous studies have shown that mortality in critical COVID-19 patients treated with glucocorticoids was lower than in other patients (21). The use of corticosteroids can be associated with complications such as hyperglycemia, secondary infections, neuromuscular disorders (35), or the risk of activating latent infections such as herpes, strongyloidiasis, tuberculosis, and hepatitis B virus. Therefore, during the use of corticosteroids, control of blood sugar and careful monitoring of the patient's clinical condition for the use of alternative drugs or the use of broad-spectrum antibiotics is significant (31).
3.3.4. Anticoagulant Therapy
SARS-COV-2 infection is usually associated with inflammation and prothrombotic condition, which is manifested by an increase in fibrin, fibrinogen, fibrin degradation products, D-dimer, and deterioration in the patient's clinical condition (1). However, the dose of anticoagulant cannot be changed based on D-dimer alone. Prophylactic anticoagulants can be used in patients with coagulation disorders without active bleeding. In such cases, if the platelets are less than 25,000 per μL or fibrinogen is less than 50 mg/dL, these drugs should be discontinued. The use of anticoagulants to prevent venous thromboembolism (VTE) is on the agenda of many treatment teams. In hospitalized COVID-19 patients, heparin is used in doses of 5000/SC/BD or TDS (in BMI ≥ 40: heparin 7500/IU/SC/TDS) and enoxaparin 40 mg/SC/daily (in BMI ≥ 40: Enoxaparin 40 mg BID). Also, in critical cases, prophylaxis with heparin at a dose of 7500 mg/SC/TDS or enoxaparin at a dose of 40 mg/BD/SC can be effective.
Patients with thromboembolic events (VTE) confirmed by imaging (CT angiography and Doppler ultrasound for DVT) are treated with therapeutic doses of anticoagulants. Also, in patients with underlying diseases treated with anticoagulant and antiplatelet therapies, treatment with anticoagulants can be continued after diagnosis of COVID-19 (20, 36). In general, the role of anticoagulants as a therapeutic dose in the treatment of hospitalized COVID-19 patients has not yet been conclusively confirmed and should be used as a clinical trial.
3.3.5. IL-6 Inhibitors
Tocilizumab, an IL-6 blocker, is administered at a dose of 4 - 6 mg/kg /IV (400 mg/IV in 60 kg adult weight) per 100 ml NS per hour in newly admitted patients requiring supplemental oxygen through noninvasive ventilation or high flow nasal cannula (in the first three days of hospitalization), or patients who are in the first three days of admission and are transferred to the intensive care unit within 24 hours and need to receive oxygen through a high-flow nasal cannula, non-invasive ventilation, and invasive mechanical ventilation (35). It is also used in combination with dexamethasone in patients with elevated levels of inflammatory markers (CRP ≥ 75 mg/L).
The use of tocilizumab is prohibited if any of the following conditions are present (26): (1) severely immunosuppressed patients using biological immunomodulatory; (2) patients with alanine aminotransferase more than 5 times upper limit normal; (3) patients at risk for gastrointestinal perforation; (4) bacterial, fungal, or viral infections; (5) absolute neutrophil count < 500 cells/µL; (6) platelet count < 50,000 cells/µL; (7) patient sensitivity to tocilizumab; (8) continue the treatment process.
After performing the above treatments, patients are discharged if they have the following conditions, receiving the necessary treatment-care instructions: (1) improving clinical symptoms
SpO2 in patients with chronic pulmonary disorders more than 88% and in people without chronic pulmonary disorders more than 90%; (2) decreased respiratory rate; (3) reduction in CRP; (4) improving CXR or CT-scan results.
If the patient's condition does not improve, treatment will continue according to the following algorithm (Figure 1):
3.3.6. Interferon-β
Interferons are among the cytokines with an antiviral activity considered as potential treatments for COVID-19 (37). This drug is in the form of a clinical trial with the main treatment line for the treatment of patients with mild to moderate COVID-19 (less than 10 days from the onset of symptoms) with a dose of 12 million units subcutaneous to five doses every other day for three to five doses (38). This drug is prohibited when the patient is being treated with other immunomodulatory or chemotherapy drugs (39).
3.3.7. Intravenous Immunoglobulin (IVIG)
IVIG is usually given at a dose of 25 g/day for three days in addition to the above treatment and the form of a clinical trial. It can be used for the treatment of patients with COVID-19 with symptoms, including SpO2 < 90% in FiO2 > 30 - 40%, and bilateral pulmonary infiltration (40).
3.3.8. Convalescent Plasma Therapy
The use of improved patient plasma is an adjunctive therapy for treating severe COVID-19 patients who do not respond to other therapies, which has also been approved by the FDA (41). Due to its high content of antibodies, this plasma can play a potential role in inhibiting the virus and improving the inflammatory response. Plasma therapy is performed by transferring one unit of plasma (500 to 650 mL) within 4 h (and another unit 24 h later if necessary). During the plasma transfer to the patient, the patient's clinical symptoms are carefully evaluated (26).
3.3.9. Cell Therapy
The multipotency and self-renewability of mesenchymal stem cells have led to the use of this type of cell to treat lung tissue damage and inhibit inflammatory responses in patients with COVID-19 (42). Also, since these cells lack the ACE2 receptor, they are thought to be immune to SARS-COV-2 infection (43). Currently, limited information is available on the effectiveness of this type of treatment in curing patients with COVID-19. Studies have shown that cell therapy can be associated with complications such as tumor growth, exacerbation of infections, thrombus formation, and site reactions and the FDA has warned about the vulnerability of these patients to stem cell therapy (44). However, some studies on the use of mesenchymal stem cells in treating COVID-19 have been associated with promising results. Therefore, cell therapy for COVID-19 patients should only be used as a clinical trial.
3.3.10. Anti P D-therapy
Because the expression of programmed cell death protein 1 is associated with suppression of the inflammatory activity of T lymphocytes and weakening of the immune system, blocking the inhibitory pathway of this protein and its ligand (PDL-1) restores the ability to proliferate, secreting cytokines of TCD8 + cells. As a result, PD-1 inhibitor drugs can kill the virus (45).
3.3.11. Hemoperfusion
Hemoperfusion is a treatment that can be used if there is evidence of increased inflammatory markers or severe hemodynamic disorders in COVID-19 patients (46). During this procedure, a large amount of the patient's blood is transferred through an absorbent material in order to remove toxic substances. By absorbing cytokines, the hemoperfusion cartridge prevents them from binding to the alveoli and endothelium of blood vessels, thus preventing the progression of acute respiratory distress syndrome (ARDS). Contraindications to hemoperfusion include conditions such as pregnancy, sensitivity to the cartridge compounds used, thrombocytopenia, and severe obesity (47, 48).
3.4. Criteria for Admission in Intensive Care Units (ICU)
If patients have one of the following symptoms, they will be admitted to the intensive care unit (49-51): (1) patients whose oxygen saturation does not exceed 90% despite receiving non-invasive ventilation and high-flow oxygen therapy; (2) PaO2 ≤ 60 mmHg; (3) no response to oxygen therapy; (4) severe respiratory distress; (5) acid-base disorders; (6) hemodynamic instability.
3.5. Hospital Discharge and Follow Up
Patients with chronic pulmonary disorders with SpO2 more than 88% and patients without chronic pulmonary disorders with SpO2 more than 90% in ambient air (as mentioned above) who have sustained vital signs can be discharged from the hospital. The process of continuing treatment and care at home is taught to the patient by the treatment team. If necessary, the patient will go to the hospital for follow-up at the time prescribed by the physician.
4. Conclusion
This article has included an evidence-based guideline regarding COVID-19 diagnosis, treatment, and patient follow-up to present best practices in this regard.