1. Context
Inflammation is a local, protecting reaction to injury or microbial invasion. It must be controlled precisely since insufficiencies or excesses of the inflammatory response could cause morbidity, shorten lifetime and decrease quality of life (1). Inflammation is generally characterized by redness, edema, fever, and pain, which might bring about loss of organ function dealing with the involved tissue (2). Inflammation could be classified into two groups, acute and chronic inflammation, and each possesses certain types of treatments. Drugs such as steroidal anti-inflammatory drugs (SAID) and non-steroidal anti-inflammatory drugs (NSAID) are usually utilized for the treatment of acute inflammatory complaints (3, 4). These medications have not been fully efficient for treating chronic inflammatory complaints; they temporarily suppress the diseases. In addition, patients suffering from adverse effects of the mentioned drugs, such as gastrointestinal disorders, ulcers, and liver disease, will increase, consequently (5). Therefore, there is a need for different and safe anti-inflammatory medicines, and one of the current and essential research candidates is herbal products (6). Unlike the drugs (SAID and NSAID), which pose an impact by a single component, medicinal plants might act by multitudes of different active compounds. The synergistic effect may occur and impress the targeted elements of the molecular pathways (7). Most developed or developing countries have herbal pharmacopeia separately; in addition to the usage of herbal products in the treatment procedure by the health care team, there is the more widespread usage of unofficial herbal treatments among the people in the society. Their use is grounded either in traditional medicine or modern medicine (8). There is a prevalent tendency to use medicinal herbs for the treatment of disorders (9). Since ere could be some potential side effects, contraindications, and severe drug-herb interactions through the use of herbal medicines, it is necessary to control and monitor the safety and effectiveness of those medicinal herbs (10, 11).
In this review, among the numerous herbal products used in traditional medicines to manage disorders, plants of Symphytum L. genus were selected to focus on anti-inflammatory effects. Symphytum L. spp. has centuries-old diverse usages in traditional medicine. Interestingly, the origin of the Latin name of Symphytum L. is divided into two syllables; symphis refers to the growth and strengthening of the bones, and phyton means the plants that were believed to help wounds healing, referring to its usage in the old days. Several randomized controlled studies have demonstrated the effectiveness of products made from different species of Symphytum L. for the oral and topical treatment of inflammatory disorders such as wound healing, swelling of joints and muscles in rheumatoid arthritis, acute myalgia in the back, sprains, and strains after sports accidents (12). The roots of S. officinale L. have a high reputation as a natural remedy in traditional medicine, especially in Europe, since millenary years ago (13). S. officinale L. has been well accepted in Northern America, so Native Americans admired the healing effects and included S. officinale L. roots in their main therapeutic armamentarium (14). There are internal and external applications for roots and leaves of Symphytum L. spp. External dosages forms are as an ointment, compress or alcoholic digestion, and internal dosages forms are as herbal tea or tincture (15).
In this review, we tried to assess the reports to determine the Symphytum L., genus’s prominence in the treatment of the inflammation process, elucidate the existing mechanisms of action, and compare the anti-inflammatory effects of Symphytum L. genus with Anchusa L., Borago L., Lithospermum L., Nonea L., Pulmonaria L. other genera belong to the Boraginaceae family. There are several genera of the mentioned family with valuables anti-inflammatory properties.
2. Search Strategy
We gathered about 200 detailed, evidence-based data through scientific papers published online from inception 2000 to 2021, and the number of studies included was 133. The search terms were, “comfrey AND clinical therapeutic”, “Boraginaceae AND inflammation,” “ inflammatory diseases”, “Boraginaceae”, “Anchusa L. ”, “Borago L. ”, “Lithospermum L. ”, “Nonea L. ”, and, “Pulmonaria L. ” in the title and keywords and the language was English. A wide range of databases, including Science Direct, PubMed/Medline, and Scopus search engines, were searched to identify relevant reports.
3. Inflammation
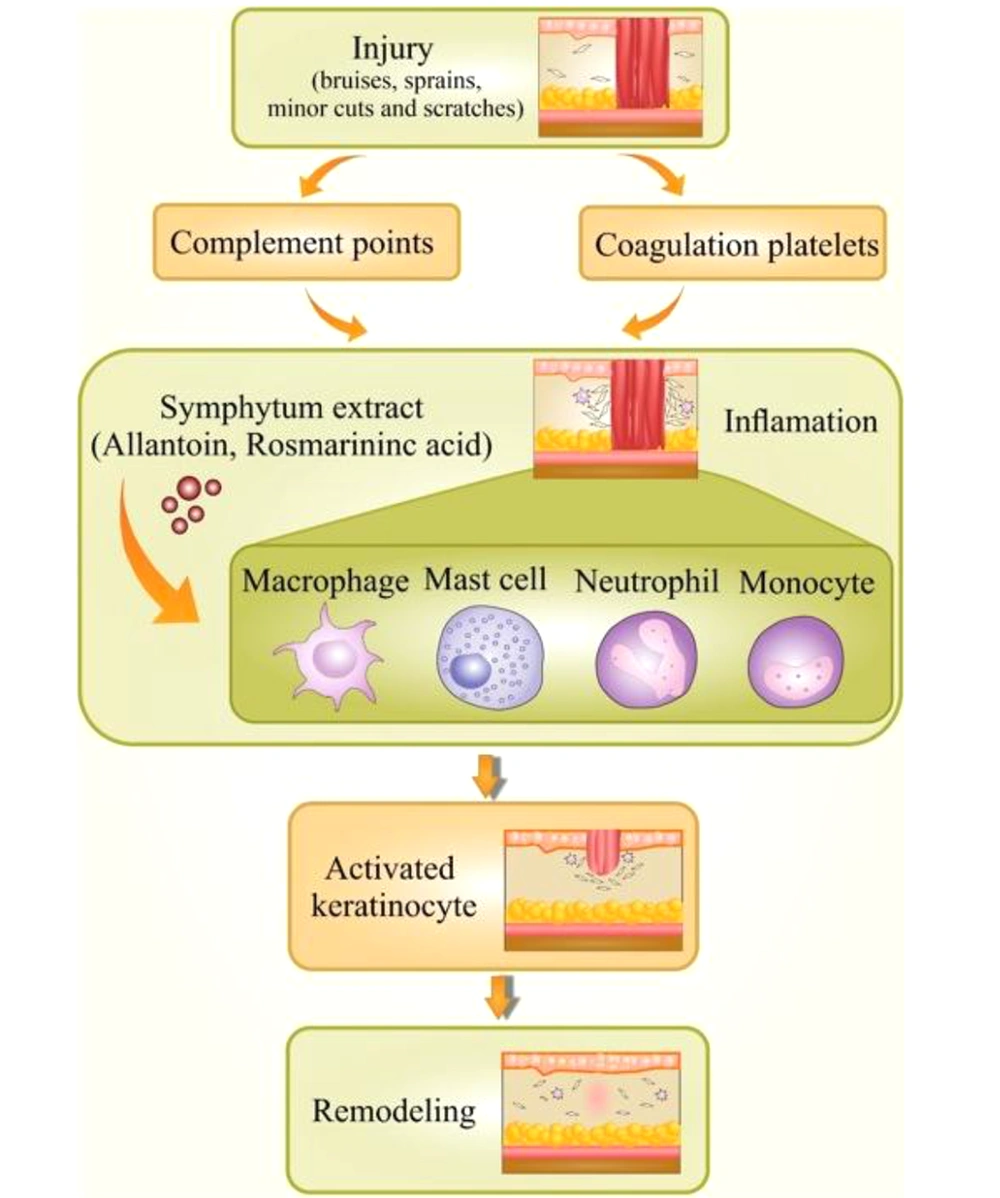
Not surprisingly, inflammation is responsible for contributing to half of the world’s global burden of disorders. Chronic inflammatory diseases are one of the most significant causes of death globally (16, 17). It is an incredibly dynamic process that responds to a various stimuli containing chemical, physical or microbial injury. Inflammation is a valuable reaction in body defense, signifying the first response to injury (18). A controlled inflammatory response is an excellent complex process that acts over a mechanism bringing about the clearance of injuries (19). As far as we know, the initiation of the inflammation process is mediated via signals and controlled through individual mediators. Different types of mediators and cells are implicated in the process of inflammation, which could regulate cell migration, chemotaxis, and proliferation in a significantly coordinated way (20, 21) Commonly, an incidence causes damaged cells to release chemicals, including histamine, bradykinin, and prostaglandins. These chemicals trigger inflammation and activate the endothelial cells in blood vessels close to the site of trauma. This action lets adhesion and transmigration of leucocytes from the blood circulation inside the hurt tissue. Acquisition of this ability, endothelial cells convey a wide-ranging variety of pro-inflammatory genes such as cell adhesion molecules (P-selectin, E-selectin, VCAM-1), cytokines, and chemokines [interleukin IL-1, IL-6, IL-8], enzymes (iNOS, COX-2, SOD), etc. (Figure 1) (22).
Inflammation may turn into a chronic condition that leads to the damage of the body structures and tissues due to the generation of potent biochemical mediators; chronic inflammation is extended for several months or even years. Frequently, the effects of chronic inflammation could be different from the impacts of acute inflammation of the hurt tissue, and the body's ability to heal the damage of chronic and acute inflammation is different (18). Additional responses of the body to the inflammation mediators might be related to chronic inflammation (23).
3.1. Inflammatory Markers
The inflammation process and its molecular basis have led to identifying inflammatory markers. These markers might suggest newfound therapy targets in the management of inflammatory-based diseases (24). Conditions with a prominent initiation of the inflammatory markers are placed into three main groups: (1) infections; (2) some hematological malignancies; (3) and autoimmune diseases. Specific proteins are regularly released from the inflammation site in-vivo, circulate in the bloodstream and bring about the whole involvement organ in a specific part of the body. Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), plasma viscosity (PV), ferritin, and fibrinogen are leading acute phase markers in the inflammation process (25). Detection and measurement of these inflammatory markers through various blood tests are frequently utilized to reveal the acute phase of inflammation that might be a sign of specific diseases and follow up the recovery process, seeing as the crucial role of these markers in treatment response. , The amount of these inflammatory markers can also be used as a universally accepted but non-specific test of severe diseases such as rheumatoid arthritis and systemic vasculitis (26). As far as we know, the marker “CRP” is signified to be principally beneficial in detecting bacterial infections. The marker “ESR” is usually revealed to the plasma viscosity, the test measures the rate of red blood cells and erythrocytes, and this marker is also independent of sex or age (27). Several reports in ethnopharmacological studies have been conducted about the effectiveness of herbal medicines in the inflammation process due to the interruption of inflammatory markers (28, 29).
4. Symphytum L.
Plants from Symphytum L. spp. are common inhabitants of humid meadows and edge of lake parts of Europe, Asia, and America, belonging to Boraginaceae. The genus Symphytum L. is also known worldwide as comfrey, knitbone, bruise wort, and slipprty wort (30, 31). Symphytum L. includes about 40 species as the most famous ones are: S. officinale L., S. x uplandicum Nyman L., S. asperrimum L., S. pregrinum ledab L., S. kurdicum L., S. caucasicum Bieb L., S. asperum Lepech L., and S. tuberosum L. (32, 33). S. officinale L.is the most renowned member of this genus that is repeatedly recorded in scientific reports with the common comfrey name and is well-known for its anti-inflammatory property (31, 34). Different therapeutic activities of Symphytum spp. have been reported to several phytochemicals, like; Allantoin, phenolic, glycopeptides, polysaccharides, and pyrrolizidine alkaloids (14). Except for Symphytum L. genus of Boraginaceae other genera from this family were reported for their anti-inflammatory effects (35). Other species of family-like, are cultivated worldwide and native to Europe, Asia. Anchusa strigosa L. was used as an antiulcer, wound healing, antirheumatic, and antiarthritis (36, 37). Anchusa L. genus contained phytochemicals such as pyrrolizidine alkaloids, tannins, triterpenes, and polyphenols (38). The genus Borage seeds oil is the substantial herbal resource of the gamma-linolenic acid (30% - 40%) that is prescribed as an anti-inflammatory agent (39). Borage seed extract is also used to treat diabetes, arthritis, and autoimmune disorders (40). The genus Lithospermum contained shikonin, shikalkin, pyrrolizidine alkaloids, flavonoids, and polyphenols (41). It was used as burns healing, antimicrobial and, anti-inflammatory agent (42). The genus Nonea L. has been traditionally used in the treatment process of diabetes respiratory diseases and was used as a wound-healing agent and anti-inflammatory agent (43). It has been established that the genus Nonea L. is the rich source of polyphenols, alkaloids, and fatty acids (44). The genus Pulmonoria L., an herbal medicine, was used against respiratory and urinary disorders (45, 46). It has been reported that Rosmarinic acid, flavnoides, and phenolic compounds were the active ingredients in this genus (47). The genus Echium L. is mainly used as a sedative, anti-inflammatory agent, and anxiolytic possession, treating disorders including fissures of the hands and snakebites (48). It has been established that the Echium genus contained phytochemicals like naphthoquinones, flavonoids, terpenoids, and polyphenols (49).
5. Phytochemicals and Anti-inflammatory Properties of Symphytum L. spp.
Chronic inflammatory systemic diseases like rheumatoid arthritis are considered whole lifetime debilitating disorders, increasing the mortality rate bringing about high costs in healthcare for the patients and public health organizations (50). Accordingly, pharmacotherapy in these inflammatory disorders is one of the vital healthcare issues. Herbal products hold a unique place among the public and researchers, revealing appealing natural sources for detection and proceeding with novel drug candidates. Regarding the natural products, different constitutes and preparations from S. officinale L. have been extensively used to treat inflammatory disorders such as painful muscle and joint illnesses bone and wound healing (18). In a study by Thibane et al., leaves of S. officinale L. were used to stimulate healing, reduce inflammation, resulting in the alleviation of joint and muscle disorders and more facilitated functional improvements (51). In a randomized, placebo-controlled, double-blind study by Koll et al., this genus was also renowned for its effectiveness in treating gonarthrosis, acute lower and upper back pain, and blunt injuries (52). S. officinale L. has oral and topical preparations (53), used for its analgesic and anti-inflammatory activities, validated by modern clinical studies; however, the molecular base of action is still elusive (18, 54). Some scientific shred of evidence is explaining the pathogenesis of inflammation and inflammatory diseases, underlining the role of the Symphytum L. genus in this process (5, 24). A reputable source, European Scientific Cooperative on Phytotherapy Monograph (ESCOP), introduced a monograph for S. officinale L. discussing some properties of this plant. Specifically, it has been indicated that the roots have beneficial diverse therapeutic indications such as discolorations and wound healing, strains recovery, osteoarthritis, tend vaginitis, epicondylitis, arthritis, knee joint injuries, skin inflammation, tendinitis syndrome, non-active gonarthrosis, mastitis, insect bites, and fractures, proved by numerous clinical trials (55, 56). Although S. officinale L. is the most acknowledged species in this genus, other species represent significant bioactivities like S. x uplandicum L. (Russian comfrey) with wound healing effect, and another plant is S. caucasicum L. with burn healing effects (57, 58). Diverse biological effects have been reported from the selected species of the Boraginaceae family, which especially have a reputation in the treatment process of inflammation (Table 1).
| Genus | Active Components | Biological Activity | Geographical Distribution | References |
|---|---|---|---|---|
| Symphytum | Allantoin, pyrrolizidine alkaloids, choline, tannins, rosmarinic acid, and triterpenoid saponins. | Wound healing effects, strains recovery, osteoarthritis, tend vaginitis, epicondylitis, arthritis, knee joint injuries, skin inflammation. | Europe, Asia, and America | (59) |
| Pulmonaria | Allantoin, Rosmarinic acid, flavenoides, and phenolic compounds. | Treating respiratory disorders and urinary disorders anti-lithiasis activities. Wound healing effects. | Europe and western Asia | (47) |
| Nonea | Phenolic compounds, pyrrolizidine alkaloids, fatty acids, flavonoid, and saponines. | Treating diabetes, respiratory disorders, and wound healing agent. | the Mediterranean districts | (44) |
| Anchusa | Allantoin, pyrrolizidine alkaloids, tannins, triterpenes, and phenolic compounds. | Antiulcer, wound healing, Diaphoretic, antipyretic, narcotic, antipyretic, antirheumatic, and antiarthritis. | tropical and Mediterranean districts | (38, 60) |
| Echium | Allantoin, naphthoquinones,flavonoids, terpenoids, and phenolic compounds. | sedative, and anxiolytic, treating disorders including fissures of the hands, general scratches, and snakebites. | Mediterranean, North Africa and Europe | (49) |
| Borage | Allantoin and gamma-linolenic acid | Treating multiple sclerosis, diabetes, arthritis, eczema, and autoimmune disorders. | Europe, North Africa and Asia | (39) |
| Lithospermum | Allantoin, pyrrolizidine alkaloids, shikonin, shikalkin, flavonoids, and phenolic compounds. | Wounds and burns healing, antimicrobial, and antiparasitic agent. | Native to Europe, Asia, Africa. | (61) |
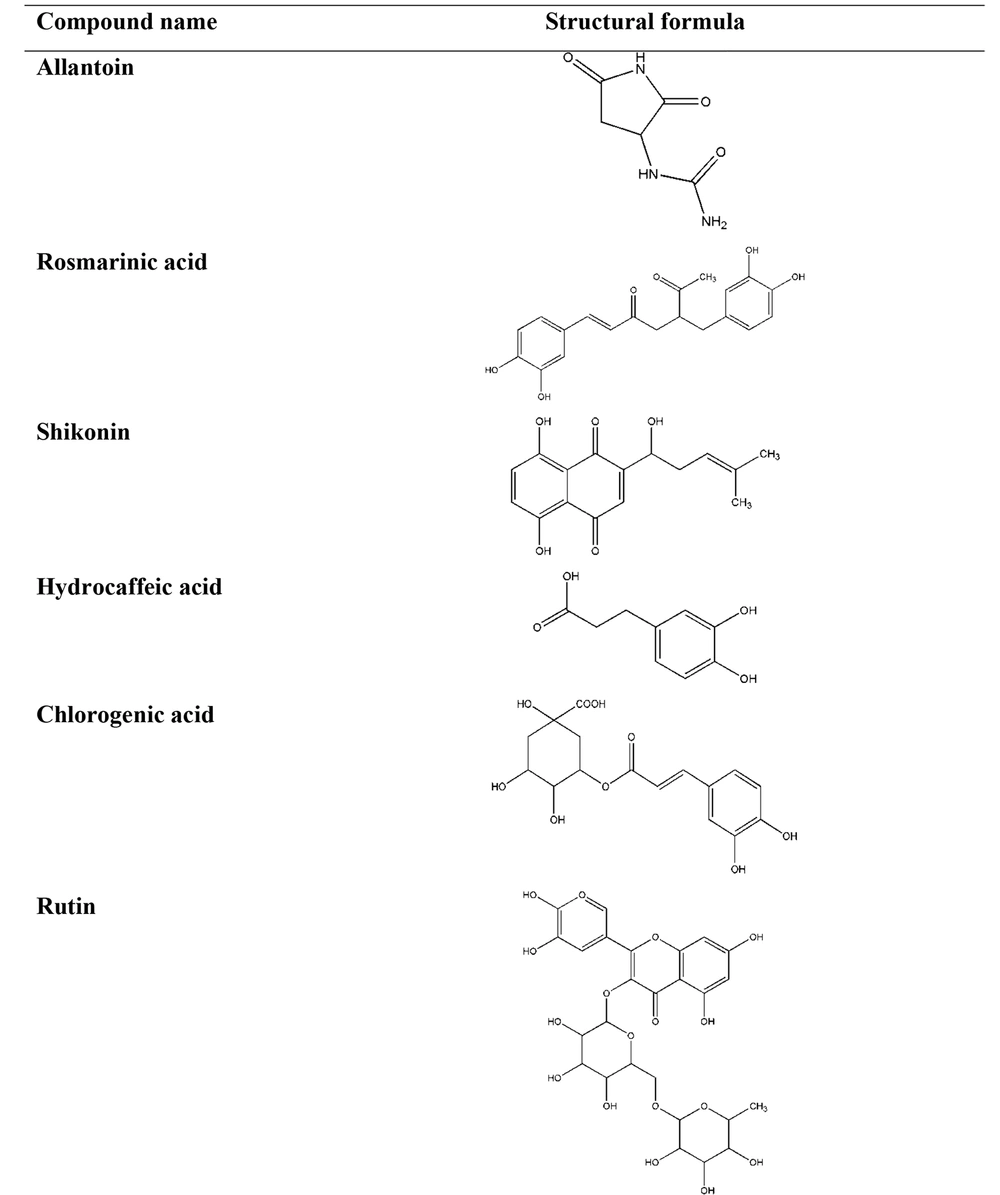
Diverse mechanisms of the anti-inflammatory preparations from the Symphytum and selected members of the Boraginaceae family L. spp. have been revealed to the constituents playing roles in these procedures (62). It has been confirmed that allantoin, choline, tannins, rosmarinic acid and its derivatives, poly[3-(3,4-dihydroxyphenyl) glyceric acid], shikonin, triterpenoid saponins, and essential oil were of the leadings phytopharmaceuticals present in these plants (63, 64). Considerably, these constituents vary depending on the plant species and special part of the herb. The chemical structures of phytochemicals, structure activity relationship (SAR), and the source of the phytochemicals have important effects on the anti-inflammatory activities of a plant (Table 2 and Figure 2) (65, 66).
| Group Compound | Compound Name | Source | Anti-inflammatory Effects | References |
|---|---|---|---|---|
| Imidazolidine-type alkaloid | Allantoin | Aerial parts and roots of Symphytum, Aerial parts of Borage, Aerial parts and roots of Lithospermum, Aerial parts and roots of Anchusa, Aerial parts and roots of Echium, Aerial parts and roots of Nonea, Aerial parts and roots of Pulmonaria | stimulating cell proliferation, improving regeneration of damaged tissues | (67, 68) |
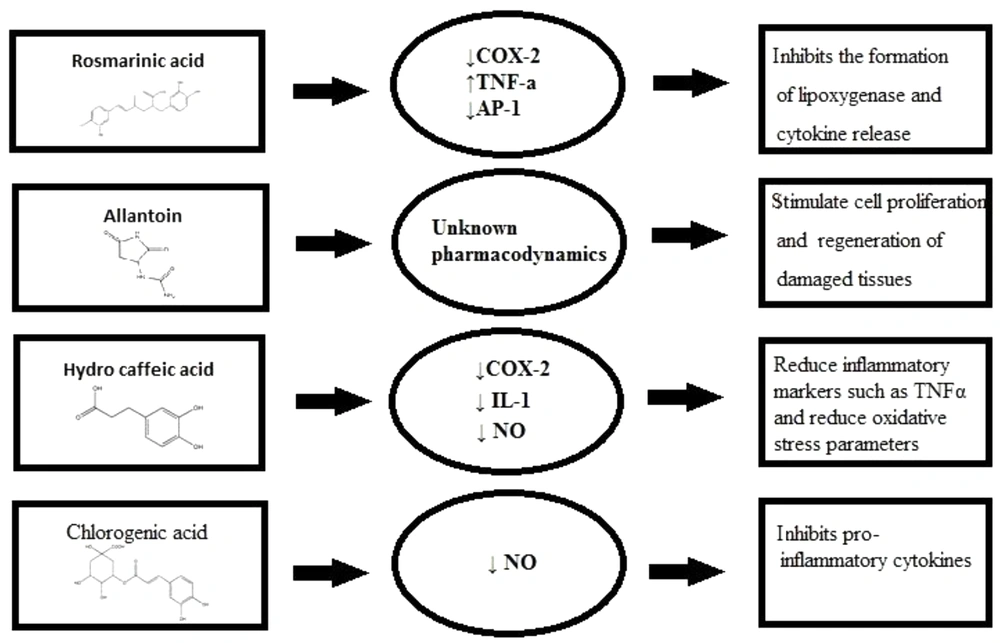
| Phenolic acid | Rosmarinic acid | Aerial parts and roots of Symphytum, Aerial parts and roots of Borage, Aerial parts and roots of Lithospermum, Aerial parts and roots of Anchusa, Aerial parts and roots of Echium, Aerial parts and roots of Nonea, Aerial parts and roots of Pulmonaria | inhibits the formation of pro-inflammatory mediators, inhibits the formation of lipoxygenase, inhibits the formation of 5-HETE, inhibits cytokine release | (67, 69) |
| Naphthoquinone | Shikonin | Roots of Symphytum, Roots of Lithospermum, Roots of Echium | suppresses the transcriptional activation of the TNF-α promoter | (67, 70, 71) |
| Phenolic acid | Hydrocaffeic acid | Aerial parts and roots of Symphytum, Aerial parts and roots of Lithospermum, Aerial parts and roots of Echium, Aerial parts and roots of Nonea | inhibited the release of IL-1β | (67, 72) |
| Phenolic acid | Chlorogenic acid | Aerial parts of Symphytum, Aerial parts of Lithospermum, Aerial parts of Nonea, Aerial parts and roots of Pulmonaria | inhibits NO, inhibits pro-inflammatory cytokines | (67, 73-75) |
| Flavonoid | Rutin | Aerial parts of Symphytum, Aerial parts of Lithospermum, Aerial parts of Anchusa, Aerial parts of Echium | suppresses the production of TNF-α, suppresses interleukin 6, suppresses the activation of NF-κB | (68, 73, 76) |
Allantoin, 5-ureide-hydantoin, is a metabolic compound of uric acid oxidation stimulating cell proliferation and improving regeneration of damaged tissues, while the compound choline decreases capillary permeability and, as a result, acts as an anti-oedemateous (77, 78). It has been reported that the quantity of allantoin in the selected species was in the range of 0.6 - 11.8 mg.g-1 air-dried matter in the aerial parts and 0.1 - 34.9 mg.g-1 air-dry matter in the roots. The maximum amount of this compound was detected in Echium italicum L. aerial parts (9.59 ± 1.96 mg.g-1) and roots (34.89 ± 10.4 mg.g-1), whereas in S. officinale aerial parts, it was reported as 9.38 ± 2.72 and in roots, it was 25.77 ± 17.02 mg.g-1 (67). However, allantoin's molecular mechanism of action and its pharmacodynamic have remained unknown (79). Choline increases tissue perfusion through vasodilatation and supports the clearance of inflammations mediators from the involved tissue (78). Choline shows its anti-inflammatory effect by triggering alpha-7 nicotinic receptors and reducing cytokine production in macrophages (80). Rosmarinic acid, a phenolic compound in Symphytum L. spp., inhibits the formation of pro-inflammatory mediators, lipoxygenase, and 5-HETE also expresses antiphlogistic activity with no relative activity upon prostaglandin synthesis (81, 82). Moreover, rosmarinic acid binds to T-cells and blocks signaling pathways to the nucleus resulting in cytokine release inhibition like IL-1 that would be used in treating autoimmune diseases (83). The rosmarinic acid quantity in the selected plant species was in the range of 1.2 - 36.6 mg.g-1 air-dry matter in the aerial parts and 1.3 - 27.0 mg.g-1 air-dried matter in the roots. The maximum amount of this compound was detected in Pulmonaria mollis aerial parts (36.6 ± 1.2 mg.g-1) and roots of Anchusa undulata (27.0 ± 5.65 mg.g-1). In aerial parts of S. officinale, rosmarinic acid quantity was detected as 4.5 ± 1.5, and in roots, it was 7.1 ± 2.63 mg.g-1. Compared to S. officinale, the amount of rosmarinic acid was much more in the other species, S. cordatum, in aerial parts (12.4 ± 1.3) and less in the amount in the roots (7.19 ± 0.75) (67). Allantoin, rosmarinic acid, and choline were believed to be the most responsible components for the anti-inflammatory and wound-healing properties in the mentioned plants (84). Phenolic compounds of S. officinale L. have been ascertained to be used as an anti-inflammatory agent in experimentations both in-vitro and in-vivo (85). A polysaccharide; Poly[3-(3,4-dihydroxyphenyl)glyceric acid], showed antioxidant and anti-complement effects putting a stop to tissue damage that would be beneficial in several pathological disorders (86).
S. officinale L. has anti-inflammatory properties that could be attributed to inhibition of IL-1β release, significantly (87). Shikonin suppresses the transcriptional activation of the TNF-α promoter concluded inhibiting the binding of Transcription factor II D (a complex of proteins that binds to a TATA series on the DNA) complex to TATA box (a sequence of DNA) within the basal transcription machinery and so the consequent expression of the TNF-α protein. It is well accepted that shikonin possesses valuable therapeutic profits for skin-related inflammatory diseases and conceivably in inflammatory disorders attendant with increased TNF-α mediators (88). There are various molecular mechanisms accredited in the treatment of inflammations commended to describe their functioning mechanism, containing the targeting of various intracellular signaling pathways provoking through Nrf2, MAPK, NF-κB, PPAR, and AP1 (Figure 3) (20).
NF-κB signaling regulated several innate and adjustable immune functions. It is believed that NF-κB is a transcription factor that adjusts pro-inflammatory cytokine gene transcription. In the study, the roots of S. officinale L. were extracted by hydroalcoholic; solution. The mucilage depleted fraction impaired the interleukin-1 (IL-1) through induction of pro-inflammatory markers, an expression containing E-selectin, VCAM1, ICAM1, and COX-2. Data afforded evidence that S. officinale L. obstructed NF-κB signaling at two different stages: at first, it reduced the activation of IKK1/2, and later degradation of IκBα, and secondly, it inhibited the NFκB p65 nucleo-cytoplasmatic transporting and transactivation (18, 26). There is another established mechanism for diverse anti-inflammatory effects of Symphytum L. spp. through the two cyclooxygenase isoforms in charge of several signs of inflammation such as vascular disorders and pain. Logically, COX-1 and COX-2 are the main enzymes in the arachidonic acid metabolism pathway resulting in the synthesis of prostaglandins. The inhibitory action of the Symphytum L. spp. was determined to be specifically on COX-2, with no effect on COX-1 enzymatic activity. Of note, no direct inhibitory activity on the enzymatic activity was detected, but the expression of COX-2 itself was powerfully blocked (18, 89). Various genera of the Boraginacea family inhibits numerous inflammatory factors (Table 3).
The aqueous extract of S. officinale L. aerial parts stimulated peroxisome proliferator-activated receptor (PPARs) and down-regulated E-selectin (expressed on endothelial cells after activation by IL-1 or TNFα and played an essential role in inflammation) mRNA and IL-8 (15, 99). The wound healing activity of S. officinale L. leaves was evaluated using three preparations via carbomer gel, glycero-alcoholic solution, and emulsion/soft lotion upon open wound rat model, using allantoin as the positive control. The results showed that the emulsion induced healing of the tissue injury and proliferation in collagen deposition from 40% to 240% and reduced cellular inflammatory infiltration by 3 - 46% (15, 99, 100). Polysaccharides like poly[3-3,4-dihydroxyphenyl) glyceric acid] from S. officinale showed the ability to deactivate the formation of active oxygen species (AOS) that are molded by activated polymorphonuclear neutrophils (PMN), performing a significant role in the protection of the body from invasive microorganisms (101). The tissue was threatened when PMN initiated extra AOS production (102). When AOS rises, the enzyme xanthine oxidase (XO) catalyzes the oxygen transformation into a superoxide anion, causing tissue damage. It was concluded that the binding of superoxide anion formed by activation of PMN and by XO was essential for healing wounds and treating inflammations (103). Another study assessed the effects of hydroalcoholic extract on healing osteoarthritis pain on 200 patients divided into two groups. One group used S. officinale L. extract cream, and the other applied placebo cream. The results showed that the patients who applied S. officinale L. cream rated their pain 16 points lower than the other group in a short time (104, 105). Elsewhere, the effectiveness of the ointment of S. officinale L. extracts compared to diclofenac gel was evaluated in an observer-blinded study reporting the curve (AUC), the tenderness, and pain assessment at rest, movement by the patient, swelling, and ankle movement. According to the statistical data, the difference between the two groups was significant, and the benefit of the herbal ointment in excess of the diclofenac gel in the treatment process of distortions was observed. The S. officinale L. products were suggested as a safe and effective alternative to the standard topical treatments (Table 4) (5, 106).
| Species | Anti-inflammatory Activity | Active Components | References |
|---|---|---|---|
| Symphytum officinale L. | Wound healing effects, Treating swelling of muscles, Treating arthritis, Treating sprains, contusions and strains after accidents | Allantoin, Rosmarinic acid, Hydro caffeic acid, Chlorogenic acid | (107-109) |
| Symphytum asperum Lepech. | Treating fractures and strains, Treating thrombophlebitis, Treating rheumatism , Treating gout, Wound healing effects | Poly [3-(3, 4-dihydroxyphenyl) glyceric acid], Caffeic acid, Rosmarinic acid, Chlorogenic acid, Salvianolic acid | (110) |
| Symphytum caucasicum | Burning healing effects, Wound healing effects | Poly [3-(3, 4-dihydroxyphenyl) glyceric acid], Allantoin | (63, 111) |
| Symphytum cordatum | Wound healing effects | Allantoin, Rosmarinic acid, Hydrocaffeic acid, P-hydroxybenzoic acid | (14, 67) |
| Symphytum × uplandicum Nyman | Wound healing effects, Treating swelling of muscles and myalgia, Treating arthritis, Treating sprains, contusions and strains after accidents, Treating joint distortion | Allantoin | (58, 112) |
| Symphytum anatolicum | Wound healing effects, Treating sprains and bruises | Caffeic acid, Allantoin, Chlorogenic acid, Rosmarinic acid, Isoquercitrin, Rutin, Hyperoside, Salvianolic acid C | (72, 113) |
6. Safety of Symphytum L. spp.
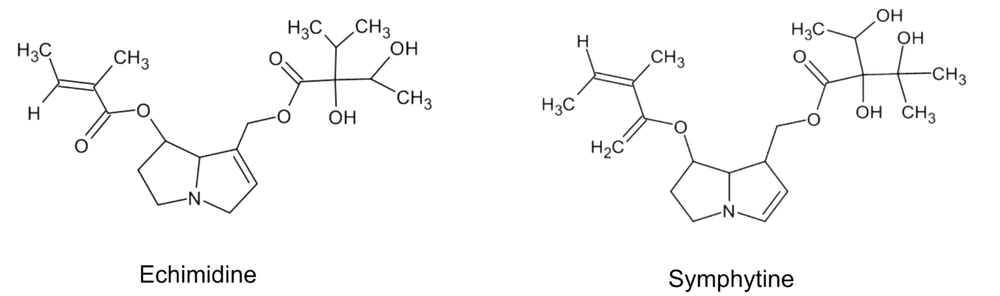
Toxicity is a crucial concern in deciding to choose a treatment procedure for a certain kind of disease. The safety of an herbal medication depends on the roots of administration, such as internal or external forms (114). It is deemed that preparation of Symphytum L. spp. Moreover, other plants of the Boraginaceae family are not safe; however, it is not supported through studies that administrated high doses of different crude compounds or prepared preparations to rodents or other animals (54). There exist not much epidemiological or clinical trial studies about the products to review them and clarify the actual toxicity in the utilized human doses (115). There is a shred of evidence that this genus, among the diversity of secondary metabolites, possesses pyrrolizidine alkaloids (PA) that are accepted as hepatotoxic phytochemicals (116). PAs are naturally inactive components and safe. They are metabolically triggered in the body by hepatic enzymes, specially CYP3A and CYP2B isoforms (117). Studies showed that toxicity varies according to the chemical structure of an individual PA in-vitro (118-120). Also, additional animals or rodents do not have the same response to PAs toxicity (121). Hence, a necessary factor to consider in determining the toxicity of a plant containing PAs is that all PAs with different chemical structures are not in the same toxicity; and various plant species have other content of PAs with different quantities (122). Different PAs are found in aerial parts and roots of Symphytum L. spp. with varying degrees of toxicity like echimidine, symlandine, symphytine, intermedine, lasiocarpine, lycopsamine, symviridine, and asperumine (Figure 4) (123-126).
The reported content of PAs in certain a plant depends not only on the extracted plant part but also on the extraction method. Since the chemical structure of the PAs are semi-polar, the concentration of PAs is shallow in extraction via a universal way of steeping parts of the plant in warm water (a polar solvent) as herbal tea (127, 128). A study investigated the PAs biosynthesis in this genus, and results revealed that there were no reported prominent adverse effects when the products were used externally. However, pharmacokinetics have reported low drug absorption via cutaneous roots (129). The safety and efficacy of S. officinale L. root extract topical preparation have been validated by numerous non-interventional studies and randomized clinical trials (52).
6.1. Liver Toxicity
Symphytum officinale roots consist of about 0.2% - 0.4% PAs (116). Internal use of comfrey root extracts is traditionally used for treating; its effectiveness and safety have never been assessed so far in controlled clinical trials however internally used preparations have not been recommended and were even restricted in the USA and Canada (120). The main liver injury caused by Symphytum and PAs is a veno-occlusive disease (VOD), a non-thrombotic obliteration of small hepatic veins leading to cirrhosis and ultimately liver failure. It may occur with either acute or chronic clinical signs with portal hypertension, hepatomegaly, and abdominal pain as the main features (122). To sum up, the risk of hepatotoxicity with the medicinal herbs preparations selected in this review article during the treatment procedure is influenced by the duration of the process, the source of the plant, the quantity of consumed, and the health crises of the patient (115, 130).
It has been accepted that the products with internal usages such as tablets and capsules in Europe and America should be labeled with the warning; “products contained a relatively low concentration of PAs” (31). It is established that the toxicity of Symphytum L. spp. is because of PAs, and the anti-inflammatory effects of this genus are primarily because of allantoin, rosmarinic acid, and other constituents. We might conclude that the natural products prepared from Symphytum L. spp. that might withdraw the toxic components would be utilized with more confidence and efficiency (131). There is a part in the Committee on Herbal Medicinal Products that dealt with the safety of Symphytum L. spp. Usage during pregnancy and lactation, in which there were no sufficient data.
In conclusion, its safety during pregnancy and lactation has not been established yet. So, due to the PAs toxicity, the use of Symphytum L. spp. Products during pregnancy and lactation are not recommended (132). Plus, no drug interactions have been reported till now (133). As a final point, all the mentioned medicinal plants belong to the Boraginaceae family, which are a source of PAs with different chemical structures; according to the quantity of PAs and non-availability of data on their safety of usage, we might consider their herbal preparations safe or dangerous to use (134, 135).
7. Conclusions
There is a strong shred of evidence, scientific reports, and clinical trials, as mentioned above, presenting an insight into the importance of Symphytum L. spp. Different pharmacological mechanisms for the effectiveness of plants of this genus in inflammation have been proposed to treat inflammatory diseases. Major Phytochemicals from Symphytum L. spp. contributed to propounding the outcome mentioned above, including allantoin, rosmarinic acid, and choline. PAs are known as hepatotoxic compounds. Several reports were reviewed, and it was established that different PAs with various chemical structures showed different toxicity levels, mainly depending on the administration route and usage duration. Nevertheless, it sounds logical and safer to utilize special extraction techniques for removing PAs from the Symphytum L. spp. herbal preparations. Overall, Symphytum L. genus, has valuable constituents for inflammation treatment, such as allantoin, various polyphenolic acids, and flavonoids; and there remains a great extent of unknowns, to seek about effectiveness of this genus with other different mechanisms of action.