1. Background
In recent years, various technologies have been used in various branches of medicine, each of which has its advantages and disadvantages. But it should be borne in mind that today the use of optimal plasma in various fields, especially medicine has made significant progress (1-3). Humans have naturally been exposed to plasma. Plasma is a type of ionized gas, and because there is always a small amount of ionization in each gas, not every ionized gas can be called plasma (4). The anti-cancer effects of cold atmospheric plasma (CAP) on various cells have so far been investigated in both in vitro and in vivo studies (5-7). CAP has potential effects on cancer cells and low cytotoxicity on normal cells, so it is known as a useful tool in the treatment of cancer. According to studies, the incidence of cancer is increasing (8-10). The selective effect on cancer cells and the lack of side effects on normal cells are features of an ideal anti-cancer therapy (11).
There is a positive correlation between a rise in the level of free radicals and the anti-cancer effects of CAP. CAP generates a lot of reactive oxygen species (ROS) that cross from the nuclear membrane and cause serious damage to DNA in cancerous cells but it has no thermal effects leading to a biological response. CAP has been shown to induce apoptotic signaling in cancer cells via the mitochondrial pathway. CAP-induced ROS causes a rise in mitochondrial membrane permeability and the release of cytochrome c (as a main pro-apoptotic protein), leading to activation of apoptosis signaling. Thus, CAP can kill cancer cells through ROS (3, 6, 12, 13). Today, the prevalence of cancer is increasing. Worldwide, oral squamous cell carcinoma (OSCC) is known as one of the most important malignancies that often occurs in the head and neck. It is also known as one of the most important types of oral cancer and tobacco and alcohol are the most important risk factors (14-16). Cisplatin is an anticancer agent commonly present in chemotherapeutic regimens used for the treatment of OSCC. Different methods for the treatment of OSCC can be problematic due to side effects on non-specific cells. So there is a major need to develop new anticancer strategies for the treatment of OSCC with a more selective cytotoxic effect on this tumor cells and preferably no toxicity on other normal body cells.
Research has shown that various compounds can induce apoptosis and increase the level of ROS in OSCC cells, and can help treat this cancer (17-20). Furthermore, cancer cells are vulnerable to free radicals (ROS) and oxidative stress. ROS can induce apoptotic signaling. So, factors that can increase the generation of ROS and induce apoptosis in cancer cells have been considered. Mitochondria are vital organelles involved in ROS generation and cell death. In recent years, mitochondria have been the focus of cancer research due to their unique properties. Various studies have shown that different compounds with impaired mitochondrial function can increase the level of ROS and apoptosis in cancer cells.
2. Objectives
This research was planned to evaluate the selective toxicity of CAP on OSCC cells and mitochondria with the possible synergistic effects of cisplatin.
3. Methods
3.1. Chemical
Rhodamine 123 (Rh 123), 2,7‐dicloroflurescein diacetate (DCHF‐DA), and caspase-3 assay kit were purchased from Sigma Chemical Co (St. Louis, MO). Cytochrome c assay kit was purchased from R&D Systems, Inc. (Minneapolis, MN). Also, other chemicals were of the highest commercial grade available.
3.2. Cold Atmospheric Plasma (CAP) Treatment
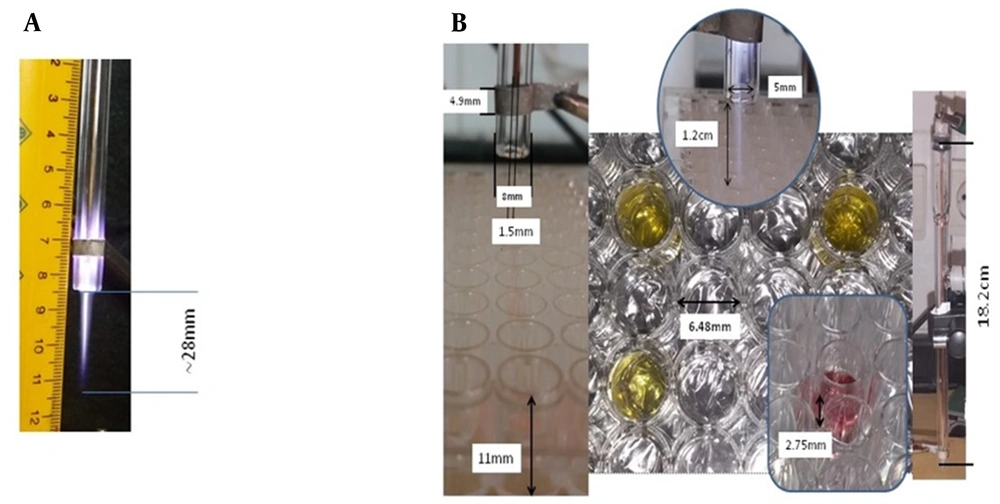
The characteristics of the CAP device and its treatment scheme are described in our previous study (Figure 1A and B, Table 1) (21). The device made at the Iran University of Science and Technology was a DBD-Like plasma jet to produce CAPP. In this research, the glass flow meter model KT800-6 with the ability to measure 1000 - 100 liters per hour with an accuracy of 40 liters per hour, has been used to control and measure the flow rate of gas. To form plasma, the applied voltage must be higher than the breakdown voltage. To use plasma to treat isolated mitochondria, it was necessary to test the power supply at different voltages and frequencies. In plasma jets that use noble gases such as helium and argon, the voltage and frequency are about kV and kHz, respectively, which are used in the treatment of cancer and the effect on DNA. In this study, the feeding gas was 99.999% pure Argon (Ar). Also, the gas pressure used in the experiments was 600 NL/h, approximately equal to 10 liters per minute. The time durations for plasma treatment were 30, 60, and 120 sec (21, 22). Our time for mitochondrial OSCC treatment was 30, 60, and 120 sec. Also, the plasma dose was calculated based on D ~ Q * V * T (Table 1). The characteristics of the CAP are shown in Table 1. CAP concentrations (1200, 2400, and 4800 a.u.) were used based on our previous study.
| Cold Atmospheric Plasma (CAP) Parameters | Values |
|---|---|
| Voltage (kV) | 4 |
| Frequency (kHz) | 40 |
| Time (s) | 30, 60, 120 |
| Plasma dose | D is the entire "plasma dosage” applied to mitochondria; Q is the flow rate of the feeding gas, V is the output voltage and T is the treatment time |
3.3. Animals
In this study, normal rats and rats with OSCC were obtained from Iran Cancer Institute. Animal care conditions (temperature 20 - 25°C; light/dark cycle 12:12 h; humidity 50 - 60%) and experiments have been performed according to the ethical standards and protocols of Shahid Beheshti University of Medical Sciences in Tehran, Iran, and our study was approved by the research ethics committee of Shahid Beheshti University of Medical Sciences with the approval code (IR.SBMU.PHARMACY.REC.1397.234).
3.4. Mitochondria Isolation
After the separation of OSCC and normal tissues, DMEM culture media was used for suspension. In the next step, the samples obtained from both groups were homogenized. Two centrifugation steps were used to isolate mitochondria. The first step, centrifugation at 500 × g for 15 minutes at 4°C and second step, centrifugation at 5000 × g for 15 minutes at 4°C (23). MTT test and cytochrome c oxidase (complex IV) assay kit were used to evaluate the mitochondrial function and the mitochondrial membrane integrity, respectively. Finally, tests were performed on mitochondria.
3.5. Evaluation of Succinate Dehydrogenase (SDH) Activity
Initially, Tris buffer was used to suspend isolated mitochondria from both groups. Then, the mitochondria obtained from both groups were exposed to CAP (1200, 2400 and 4800 a.u), cisplatin (10 µM); as a positive control; and CAP (2400 a.u) plus cisplatin (10 µM), and then incubated for 1 hour at 37°C. Then, succinate dehydrogenase (SDH) activity was evaluated after adding MTT dye (0.4%) and dimethyl sulfoxide (DMSO), and absorbance reading at 570 nm (ELISA reader) (24, 25).
3.6. Evaluation of ROS Level
DCHF-DA reagent was used to evaluate the level of ROS. Briefly, isolated mitochondria were suspended in the respiratory assay buffer and then exposed to different doses (1200, 2400 and 4800 a.u) of CAP, cisplatin (10 µM), and CAP (2400 a.u) plus cisplatin (10 µM). Then, a DCHF-DA reagent (10 µM) was added to the mitochondrial suspension and the incubation process was performed for 1 hour at 37°C. Next, the level of ROS was assessed using a fluorescence spectrophotometer (EX = 488 nm and EM = 527 nm) (21).
3.7. Evaluation of Mitochondrial Swelling
Initially, isolated mitochondria from both groups were suspended in mitochondrial swelling assay buffer (sucrose (70 mM), mannitol (230 mM), HEPES (3 mM), Tris-phosphates (2 mM), succinate (5 mM), and rotenone (1 M)). Then, the mitochondria obtained from both groups were exposed to different doses (1200, 2400 and 4800 a.u) of CAP, cisplatin (10 µM), and CAP (2400 a.u) plus cisplatin (10 µM), and then incubated for 1 hour at 37°C. Then, mitochondrial swelling was evaluated at an absorbance of 540 nm (ELISA reader).
3.8. Evaluation of MMP Collapse
Rh 123 reagent was used to evaluate the MMP collapse. Briefly, isolated mitochondria were suspended in the MMP assay buffer and then exposed to different doses (1200, 2400 and 4800 a.u) of CAP, cisplatin (10 µM) and CAP (2400 a.u) plus cisplatin (10 µM). Then, Rh 123 reagent (10 µM) was added to the mitochondrial suspension and the incubation process was performed for 1 hour at 37°C. Next, the MMP collapse was assessed using a fluorescence spectrophotometer (EX = 490 nm and EM = 535 nm) (26, 27).
3.9. Evaluation of Cytochrome C Release
The Quantikine Rat/Mouse Cytochrome c Immunoassay kit (R and D Systems, Inc., Minneapolis, MN, USA) was used for the determination of cytochrome c release.
3.10. Cell Isolation
Cell isolation was performed based on our previous study. First, the tissue samples were immersed in 70% ethanol solution for 20 s and then washed several times with PBS. In the next step, the samples were transferred to a DMEM culture medium. Tissue culture dishes were used to the seed of small tissue pieces. Then, the primary cell cultures were incubated for 2 - 3 weeks to achieve 70% - 80% confluence at 37°C with CO2 (5%) (28, 29). In the following, the culture medium was changed every 3 to 4 days. Finally, 1 × 104 cells/mL were used to evaluate the activity of caspase-3.
3.11. Evaluation of Caspase-3 Activity
In this study, the Sigma Caspase-3 assay kit (Sigma‐Aldrich) was used to investigate the relationship between exposure to CAPP at different doses (1200, 2400 and 4800 a.u) of CAP, cisplatin (10 µM); as a positive control; and CAP (2400 a.u) plus cisplatin (10 µM) on activation of caspase-3 in both groups. The concentration of p-nitroaniline released at the absorbance wavelength of 405 nm is considered an indicator of caspase-3 activation (30).
3.12. Statistical Analysis
Statics are presented as mean ± SD. All statistical tests were carry out using GraphPad Prism (GraphPad Prism software, version 6). Statistical significance set at P < 0.05. Data were analyzed using one-way analysis of variance (ANOVA) followed by the Tukey’s test.
4. Results
4.1. CAP and Mitochondrial SDH Activity
In the OSCC mitochondria, the results showed that CAP was able to reduce SDH activity at doses of 1200, 2400 and 4800 a.u. However, CAP at these doses had no effect on SDH activity in normal mitochondria (Figure 2A). Also, cisplatin (10 µM) as a positive control reduced the activity of SDH. CAP at 4800 a.u dose had similar effects to cisplatin (10 µM) on SDH activity. In addition, exposure to CAP (2400 a.u) plus cisplatin (10 µM) significantly reduced SDH activity compared to other groups (Figure 2A).
A, Cold atmospheric plasma (CAP) and succinate dehydrogenase (SDH) activity in normal and oral squamous cell carcinoma (OSCC) mitochondria [results displayed as mean ± SD (n = 3); *** P < 0.001 compared with the control group; ### P < 0.001 compared with the control group and CAP (1200 a.u and 2400 a.u); $$$ P < 0.01 compared with the control and CAP (1200 a.u, 2400 a.u and 4800 a.u) group; $$ P < 0.01 compared with the cisplatin (10 µM) group]; B, CAP and ROS level in normal and OSCC mitochondria [results displayed as mean ± SD (n = 3); ** P < 0.01 *** P < 0.001 compared with the control group; ### P < 0.001 compared with the control and CAP (1200 a.u) group; ## P < 0.01 compared with the CAP (2400 a.u) group; $$$ P < 0.01 compared with the control and CAP (1200 a.u, 2400 a.u and 4800 a.u) group; $$ P < 0.01 compared with the cisplatin (10 µM) group].
4.2. CAP and Mitochondrial ROS Level
As shown in Figure 2B, exposure of OSCC mitochondria to CAP has been associated with an increase in ROS generation. A significant increase in the level of ROS in the OSCC mitochondria was observed at all doses (1200, 2400 and 4800 a.u). In normal mitochondria, this effect due to CAPP exposure has not been reported (Figure 2B). The results showed that CAP at the highest concentration had similar effects to cisplatin (10 µM). Also, synergistic effects between CAP (2400 a.u) plus cisplatin (10 µM) have been reported (Figure 2B).
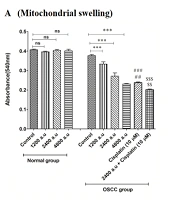
4.3. CAP and Mitochondrial Swelling
Mitochondrial swelling can be considered as one of the events in signaling apoptosis. Results indicated that there is a positive relationship between the exposure of OSCC mitochondria to CAP (1200, 2400 and 4800 a.u) and mitochondrial swelling (Figure 3A). This mitochondrial dysfunction was not observed in the normal group. Furthermore, the results showed that there was no significant relationship between CAP (4800 a.u) and cisplatin (10 µM), and plasma similar to cisplatin could cause mitochondrial swelling. Compared to the other groups, the highest mitochondrial swelling was observed in the CAP (2400 a.u) plus cisplatin (10 µM) group, which could indicate synergistic effects (Figure 3A).
A, Cold atmospheric plasma (CAP) and mitochondrial swelling in normal and oral squamous cell carcinoma (OSCC) mitochondria [results displayed as mean ± SD (n = 3); *** P < 0.001 compared with the control group; ### P < 0.001 compared with the control and CAP (1200 a.u) group; ## P < 0.01 compared with the CAP (2400 a.u) group; $$$ P < 0.01 compared with the control and CAP (1200 a.u, 2400 a.u and 4800 a.u) group; $$ P < 0.01 compared with the cisplatin (10 µM) group]; B, CAP and MMP collapse in normal and OSCC mitochondria [results displayed as mean ± SD (n = 3); ** P < 0.01 *** P < 0.001 compared with the control group; ### P < 0.001 compared with the control and CAP (1200 a.u and 2400 a.u) group; $$$ P < 0.001 compared with the control, CAP (1200 a.u, 2400 a.u and 4800 a.u) and cisplatin (10 µM) group].
4.4. CAP and MMP Collapse
As shown in Figure 3B, exposure of OSCC mitochondria to CAP has been associated with an increase in the MMP collapse. A significant increase in the MMP collapse in the OSCC mitochondria was observed at all doses (1200, 2400 and 4800 a.u). In normal mitochondria, this effect due to CAP exposure has not been reported (Figure 3B). Cisplatin at a concentration of 10 µM caused a collapse in the MMP. CAP at 4800 a.u dose had similar effects to cisplatin (10 µM) on MMP collapse. Furthermore, exposure to CAP (2400 a.u) plus cisplatin (10 µM) significantly caused a collapse in the MMP compared to other groups (Figure 3B).
4.5. CAP and Cytochrome C Release
In apoptosis, the release of cytochrome c is an important event. In the OSCC mitochondria, the results showed that CAP was able to release cytochrome c at doses of 1200, 2400 and 4800 a.u. However, CAP at these doses had no effect on cytochrome c release in normal mitochondria (Figure 4A). The results showed that CAP (4800 a.u) and cisplatin (10 µM) similarly caused cytochrome c release from the OSCC mitochondria, and no significant difference was observed between the two groups. Also, synergistic effects between CAP (2400 a.u) plus cisplatin (10 µM) have been reported (Figure 4A).
A, Cold atmospheric plasma (CAP) and cytochrome c release (1200, 2400 and 4800 a.u) in normal and oral squamous cell carcinoma (OSCC) mitochondria [results displayed as mean ± SD (n = 3); *** P < 0.001 compared with the control group; ### P < 0.001 compared with the control and CAP (1200 a.u and 2400 a.u) group; $$$ P < 0.001 compared with the control, CAP (1200 a.u, 2400 a.u and 4800 a.u) and cisplatin (10 µM) group]; B, CAP and caspae-3 activity (1200, 2400 and 4800 a.u) in normal and OSCC mitochondria. Results displayed as mean ± SD (n = 3); ** P < 0.01 *** P < 0.001 compared with the control group; ### P < 0.001 compared with the control and CAP (1200 a.u and 2400 a.u) group; $$$ P < 0.001 compared with the control, CAP (1200 a.u, 2400 a.u and 4800 a.u) and cisplatin (10 µM) group].
4.6. CAP and Caspase-3 Activity
Results indicated that there is a positive relationship between the exposure of OSCC group to CAP (1200, 2400 and 4800 a.u) and caspae-3 activity (Figure 4B). P-nitroaniline concentration levels are used to evaluate caspase-3 activity. This event was not observed in the normal group. Additionally, results indicated that there was no significant difference between CAP (4800 a.u) and cisplatin (10 µM), and plasma similar to cisplatin could cause caspase-3 activation. Compared to the other groups, the highest caspase-3 activity was observed in the CAP (2400 a.u) plus cisplatin (10 µM) group (Figure 4B).
5. Discussion
This in vitro research was designed to study the apoptotic effects of CAP and cisplatin treatment on OSCC mitochondria and cells. Common approaches to the treatment of OSCC are controversial as one of the most important types of head and neck cancer (31, 32). In past years, the use of CAP in the treatment of different malignancies has attracted the attention of researchers. Cancer treatment based on traditional methods is costly and time-consuming. It can also damage normal cells. In the treatment of cancer, the use of cold plasma is cheaper and the damage to normal cells is less (33, 34). Mitochondrial SDH activity after the application of the CAP was measured using an MTT test. It was proven that the CAP can decrease the SDH activity only of the OSCC mitochondria.
It is well known that mitochondria are involved in cell death and the generation of ROS. It has been shown that plasma anticancer activity is mediated by mitochondrial dysfunction. In addition, mitochondrial dysfunction is associated with the induction of apoptotic signaling and a rise in ROS levels (3, 35, 36). Studies have shown that the generation of ROS is effective in the treatment of cancer (37, 38). One of the mechanisms by which cold plasma induces apoptosis in cancer cells is an increase in the level of ROS in these cells (12, 39). Compared to normal cells, it should be noted that cancer cells are more sensitive to endogenous free radicals (ROS) (40). CAP treatment resulted in an increase in ROS generation in the OSCC mitochondria. It can be concluded that OSCC mitochondria are more sensitive to CAP treatment than normal mitochondria. Our results are similar to previous studies (12, 21, 39).
The collapse in the MMP and mitochondrial swelling are the hallmarks of the permeability of mitochondrial inner membrane (MIM) to various compounds. It has been shown that ROS are effective in mitochondrial membrane permeability. In addition, cytochrome c release and cell death can be affected by ROS (41-43). In this research, we found that in vitro CAP treatment increased the MMP collapse. Our results also showed that CAP resulted in mitochondrial swelling. CAP treatment had no effect on the normal mitochondria at the exposure time tested. The results of this study suggest that plasma is likely to disrupt the OSCC mitochondrial membrane potential through ROS, Eventually, it causes collapse of the MMP and mitochondrial swelling.
Subsequently, treatment of OSCC mitochondria to CAP was associated with cytochrome c release. ROS are involved in the release of cytochrome c through its effect on mitochondrial membrane permeability. This result is also in agreement with our previous study (21). Results indicated that there was a significant increase in caspases-3 activity following CAP treatment in OSCC cells. Caspase-3 is one of the important factors that play a role in cell death (44, 45). In cancer cells, the concentration of ROS plays an important role in the mechanism of caspase-mediated apoptosis (46). Based on these results, it can be suggested that CAP-induced ROS are involved in the activation of caspase-3 followed by cell death.
5.1. Conclusions
The results of the study suggest that CAP can increase the level of ROS by targeting cancerous mitochondria. Then, the permeability of the OSCC mitochondrial membrane is affected by ROS, resulting in collapse of the MMP and mitochondrial swelling. Subsequently, mitochondrial swelling was associated with the release of cytochrome c as a pro-apoptotic proteins and the activation of caspase-3. Considering the fact that combination of CAP and cisplatin significantly increased all toxicity parameters, our results suggest CAP as a promising therapeutic complementary candidate against OSCC and suggest the process for further investigation.
![A, Cold atmospheric plasma (CAP) and succinate dehydrogenase (SDH) activity in normal and oral squamous cell carcinoma (OSCC) mitochondria [results displayed as mean ± SD (n = 3); <sup>***</sup> P < 0.001 compared with the control group; <sup>###</sup> P < 0.001 compared with the control group and CAP (1200 a.u and 2400 a.u); <sup>$$$</sup> P < 0.01 compared with the control and CAP (1200 a.u, 2400 a.u and 4800 a.u) group; <sup>$$</sup> P < 0.01 compared with the cisplatin (10 µM) group]; B, CAP and ROS level in normal and OSCC mitochondria [results displayed as mean ± SD (n = 3); <sup>**</sup> P < 0.01 <sup>***</sup> P < 0.001 compared with the control group; <sup>###</sup> P < 0.001 compared with the control and CAP (1200 a.u) group; <sup>##</sup> P < 0.01 compared with the CAP (2400 a.u) group; <sup>$$$</sup> P < 0.01 compared with the control and CAP (1200 a.u, 2400 a.u and 4800 a.u) group; <sup>$$</sup> P < 0.01 compared with the cisplatin (10 µM) group]. A, Cold atmospheric plasma (CAP) and succinate dehydrogenase (SDH) activity in normal and oral squamous cell carcinoma (OSCC) mitochondria [results displayed as mean ± SD (n = 3); <sup>***</sup> P < 0.001 compared with the control group; <sup>###</sup> P < 0.001 compared with the control group and CAP (1200 a.u and 2400 a.u); <sup>$$$</sup> P < 0.01 compared with the control and CAP (1200 a.u, 2400 a.u and 4800 a.u) group; <sup>$$</sup> P < 0.01 compared with the cisplatin (10 µM) group]; B, CAP and ROS level in normal and OSCC mitochondria [results displayed as mean ± SD (n = 3); <sup>**</sup> P < 0.01 <sup>***</sup> P < 0.001 compared with the control group; <sup>###</sup> P < 0.001 compared with the control and CAP (1200 a.u) group; <sup>##</sup> P < 0.01 compared with the CAP (2400 a.u) group; <sup>$$$</sup> P < 0.01 compared with the control and CAP (1200 a.u, 2400 a.u and 4800 a.u) group; <sup>$$</sup> P < 0.01 compared with the cisplatin (10 µM) group].](https://services.brieflands.com/cdn/serve/3170b/81c0b106724076ba7a4dc747d5730483f3d86989/ijpr-124106-i001-F2-preview.webp)
![A, Cold atmospheric plasma (CAP) and mitochondrial swelling in normal and oral squamous cell carcinoma (OSCC) mitochondria [results displayed as mean ± SD (n = 3); <sup>***</sup> P < 0.001 compared with the control group; <sup>###</sup> P < 0.001 compared with the control and CAP (1200 a.u) group; <sup>##</sup> P < 0.01 compared with the CAP (2400 a.u) group; <sup>$$$</sup> P < 0.01 compared with the control and CAP (1200 a.u, 2400 a.u and 4800 a.u) group; <sup>$$</sup> P < 0.01 compared with the cisplatin (10 µM) group]; B, CAP and MMP collapse in normal and OSCC mitochondria [results displayed as mean ± SD (n = 3); <sup>**</sup> P < 0.01 <sup>***</sup> P < 0.001 compared with the control group; <sup>###</sup> P < 0.001 compared with the control and CAP (1200 a.u and 2400 a.u) group; <sup>$$$</sup> P < 0.001 compared with the control, CAP (1200 a.u, 2400 a.u and 4800 a.u) and cisplatin (10 µM) group]. A, Cold atmospheric plasma (CAP) and mitochondrial swelling in normal and oral squamous cell carcinoma (OSCC) mitochondria [results displayed as mean ± SD (n = 3); <sup>***</sup> P < 0.001 compared with the control group; <sup>###</sup> P < 0.001 compared with the control and CAP (1200 a.u) group; <sup>##</sup> P < 0.01 compared with the CAP (2400 a.u) group; <sup>$$$</sup> P < 0.01 compared with the control and CAP (1200 a.u, 2400 a.u and 4800 a.u) group; <sup>$$</sup> P < 0.01 compared with the cisplatin (10 µM) group]; B, CAP and MMP collapse in normal and OSCC mitochondria [results displayed as mean ± SD (n = 3); <sup>**</sup> P < 0.01 <sup>***</sup> P < 0.001 compared with the control group; <sup>###</sup> P < 0.001 compared with the control and CAP (1200 a.u and 2400 a.u) group; <sup>$$$</sup> P < 0.001 compared with the control, CAP (1200 a.u, 2400 a.u and 4800 a.u) and cisplatin (10 µM) group].](https://services.brieflands.com/cdn/serve/3170b/9d71e513ffd04c2091af94363b16881fbdf59cfd/ijpr-124106-i002-F3-preview.webp)
![A, Cold atmospheric plasma (CAP) and cytochrome c release (1200, 2400 and 4800 a.u) in normal and oral squamous cell carcinoma (OSCC) mitochondria [results displayed as mean ± SD (n = 3); <sup>***</sup> P < 0.001 compared with the control group; <sup>###</sup> P < 0.001 compared with the control and CAP (1200 a.u and 2400 a.u) group; <sup>$$$</sup> P < 0.001 compared with the control, CAP (1200 a.u, 2400 a.u and 4800 a.u) and cisplatin (10 µM) group]; B, CAP and caspae-3 activity (1200, 2400 and 4800 a.u) in normal and OSCC mitochondria. Results displayed as mean ± SD (n = 3); <sup>**</sup> P < 0.01 <sup>***</sup> P < 0.001 compared with the control group; <sup>###</sup> P < 0.001 compared with the control and CAP (1200 a.u and 2400 a.u) group; <sup>$$$</sup> P < 0.001 compared with the control, CAP (1200 a.u, 2400 a.u and 4800 a.u) and cisplatin (10 µM) group]. A, Cold atmospheric plasma (CAP) and cytochrome c release (1200, 2400 and 4800 a.u) in normal and oral squamous cell carcinoma (OSCC) mitochondria [results displayed as mean ± SD (n = 3); <sup>***</sup> P < 0.001 compared with the control group; <sup>###</sup> P < 0.001 compared with the control and CAP (1200 a.u and 2400 a.u) group; <sup>$$$</sup> P < 0.001 compared with the control, CAP (1200 a.u, 2400 a.u and 4800 a.u) and cisplatin (10 µM) group]; B, CAP and caspae-3 activity (1200, 2400 and 4800 a.u) in normal and OSCC mitochondria. Results displayed as mean ± SD (n = 3); <sup>**</sup> P < 0.01 <sup>***</sup> P < 0.001 compared with the control group; <sup>###</sup> P < 0.001 compared with the control and CAP (1200 a.u and 2400 a.u) group; <sup>$$$</sup> P < 0.001 compared with the control, CAP (1200 a.u, 2400 a.u and 4800 a.u) and cisplatin (10 µM) group].](https://services.brieflands.com/cdn/serve/3170b/e626155abbb3b0a9d78a22b9c91a3c2ba31019b8/ijpr-124106-i003-F4-preview.webp)