1. Context
Former outbreaks of Coronaviruses such as the Middle East respiratory syndrome (MERS)-CoV and the severe acute respiratory syndrome (SARS)-CoV have been considered as a public health threat. On January 7th, 2019 a new strain of Coronavirus , known as SARS-COV-2, was isolated, and later in December 2019 COVID-19 virus spread out as a lethal respiratory disease and World Health Organization (WHO) proclaimed a pandemic on 11th March/2020 (1, 2). According to the phylogenetic analysis, COVID-19 is different from SARS-CoV and is classified as a new beta-Coronavirus originated from bats. In addition, this virus underwent mutation and therefore it was able to infect human beings. Human-to-human transmission is another ability that these viruses acquire (3). The rapid and global outbreak of this virus along with numerous mutations and production of different mutant types (African, British, Brazilian, Indian) underlines the importance of developing effective vaccines.
More than a year has passed since the outbreak of Coronavirus, and hopes for the virus disappearance have given way to hopes of controlling it by mass production of vaccines. Since the initial outbreak, research in vaccine production began at numerous vaccine production research centers which resulted in the development of various vaccines based on theories, hypotheses, and evidence of the disease. Therefore, the number and type of corona vaccines around the world are increasing significantly, yet, only a limited number of vaccines have been approved and applied to prevent the recurrence of outbreaks. It is estimated that in the first half of 2021, all corona vaccine companies with different technologies will produce approximately 7.8 billion doses, and in the second half of this year, 12.3 billion doses of corona vaccine in the world will be available. Besides, statistics show 28 and 29 billion doses for 2022 and 2023 respectively (4). As a result, in the next few months, there will be a variety of vaccines on the market, however, there is still a question that which of these vaccines bear the minimum side effects?
Until March 21, 2021, 64 vaccines have passed the early stages of clinical trials and 20 vaccines are in the final stages of human testing (phase 3) (https://www.nytimes.com, Coronavirus Vaccine Tracker). Furthermore, eight vaccines are approved for national or limited use in several countries (Appendix 1 in Supplementary File). These vaccines include Pfizer, Moderna, Janssen & Janssen (USA), Astrazeneka (England), Sputnik V (Russia), Sinovac and Sinopharm (China), COVAX (India), etc. Here, a question can be posed concerning the differences between these vaccines, which technology do they use, and what are the pros and cons of each vaccine? This revision enlarges upon all strategies used to produce COVID-19 vaccines including virus-based vaccines (weakened live or inactivated virus), vector-based, nucleic acid (RNA/DNA)-based, and protein-based vaccines. Besides, the challenges together with the advantages of each system are explored.
The progress in bioinformatics has tremendously facilitated the development of therapeutic agents, especially vaccines in the cases of rapid outbreaks and unknown pathogens (5). Since bioinformatics imparts an effective role in genome and epitope identification, progress, and development of COVID-19 vaccines, thus this strategy is reviewed as well.
2. Bioinformatics
2.1. Reverse Vaccinology
Reverse vaccinology (RV) is the most practical arena of bioinformatics in vaccine development that examines the whole genome of viruses by computational software based on indentifying antigens/epitopes of pathogens (6-8).
Generally, RV process includes the following steps: (1) checking process of viral genome surface proteins; (2) these proteins examinations are carried out to predict the optimal epitopes; (3) ensuring the surface localization of these epitopes; and (4) testing surface proteins regarding autoimmune threats (9).
2.2. Antigen(s) Choice, Disclosure, and Optimization
New antigens are recognized by epitopes which have been identified by CD4+ T and/or CD8+ T cells. RV makes it possible, the exact determination of antigens (epitopes) as a key parameter in vaccine design based on epitopes. In conventional vaccinology, precise determination of antigen number is the principle restricting step. Computational vaccinology employments of high-efficiency information analysis and machine-learning instruments for the disclosure of essential antigen as well as antigenic potential for the discovery of ideal vaccines by monitoring the useful components and units in pathogens and pathogenesis basic organic processes (10, 11).
2.3. Prediction of B/T Cell Epitopes
A branch of Bioinformatics termed Immunoinformatics develops an arrangement of algorithms for assurance of potential B cell epitope (BCEs) and T cell epitope (TCEs). Examining the affinity of antigenic peptides for attaching to MHC particles could be a major challenge in epitope prediction (9, 12, 13).
2.4. Prediction of B Cell Epitopes
Considering in-silicoBCEs prognostication, nature and type of BCEs are determinant parameters since B cell lymphocytes are attracted to both BCEs as continuous (linear) and discontinuous (conformational, CBCEs) epitopes (14, 15). Briefly, there are fundamental principles for a precise in-silicoBCEs mapping incorporate: (1) short-length epitopes disposal (16); (2) analyzing basic properties (e.g., hydrophilicity, flexibility, and surface exposure, and solvent availability) of candidate antigens (17); (3) utilizing multimethod BCEs prediction strategies (18), and (4) comparing the web-based BCEs prediction tool results with the molecular interaction strategies such as molecular docking and molecular dynamics (MD) simulations.
The highest portion of BCEs is CBCEs (nearly 90%), nevertheless, the length of antigenic peptides binding to the paratope site of antibodies is not clear (for the most part). This is often a CBCEs-related problem since it brings about less solid (e.g., false-negative and wrong positive) results concerning the prediction of CBCEs (19). Correspondingly, the databases used for the prediction of B cell epitopes are provided in Appendix 2 in Supplementary File.
2.5. T Cell Epitope Prediction
TCEs prediction strategies are classified into two groups, namely direct and indirect strategies. Direct methods are based on the successive and structural examination of TCEs, while indirect techniques determine MHC binders. Due to the high accuracy and specificity, indirect strategies are often preferred for TCE prediction. Indirect strategies are classified into two groups: (1) MHC-I covers (CD8+ TCEs for the preparation of endogenous antigens), and MHC-II binders (CD4+ helper TCEs’ for exogenous antigen processing) (18, 20-23). In addition, databases related to the prediction of T cell epitopes are presented in Appendix 3 in Supplementary File.
3. COVID-19 Specific Vaccines
3.1. DNA Vaccines
These vaccines comprise a DNA sequence encoding immune-stimulating viral proteins or peptides. Since DNA vaccines are capable of stimulating the immune system in HIV, rabies, hepatitis B, C, and influenza conditions (24), initially this type of vaccine is studied.
Cheung and colleagues developed a DNA vaccine which encoded the most antigenic T cell epitope in N protein of SARS Coronavirus. To stabilize the MHC-1-peptide complex, the vaccine also carried the nucleotide sequence of α1, α2, and β2 domains within MHC molecule. The vaccine was administered intramuscularly at 3-week intervals three times with the concentration of 1ug DNA. Accordingly, the plasmid coding antigenic peptide was loaded on gold nanoparticles and injected by a gene gun device also co-administered with adjuvant. The results of this study revealed that the cytotoxicity level of CTLs on the cells expressing N protein of Coronavirus in vaccinated mice was 86% (25).
In another interesting study conducted by Poh et al. (2009), a DNA vaccine coding the spike protein of SARS Coronavirus, as well as arginine-glycine-aspartic acid, RGD sequence, was implemented and the vaccine was administered 4 times at 3-week intervals. After the last vaccination, cytotoxicity of splenocytes as well as antibodies’ titer, were assessed. The results revealed that the RGD sequence polarized viral immunization to cellular immunity, attributed to the binding of lymphocytes and APCs to the endothelium resulting in the lymphocytes entrance into lymphoid tissue and further stimulation of cellular immunity (1). Besides, Wang et al. (2008) developed a multi-epitope DNA vaccine encoding B cell epitopes identified in S and M proteins of SARS Coronavirus via bioinformatics analysis. This vaccine triggered polyvalent immunity as well as preventing lung injuries up to 75%. The two epitopes, S 437-459 and M 1-20, in this study, induced long-term humoral immunity and immunological memory (26).
Furthermore, Zhao et al. (2005) examined the effect of a DNA vaccine encoding SARS-Coronavirus nucleocapsid protein concerning the humoral and cellular immune response. They administered this vaccine intramuscularly at 2-week intervals three times. Correspondingly, the results illustrated that the cytotoxicity of T cells against cells expressing N protein was approximately 100 times higher than other cells. They also measured the T cell secretion of interferon- γ and interleukins in vaccinated mice which were significantly higher than the control group. Regarding humoral immunity, the level of anti-N protein antibodies was identified after two weeks as it reached 75% of the total antibodies in the sixth week following administration. What is more, the vaccine increased IgG2a concentration more than IgG1 (27). The first DNA vaccine for the MERS Coronavirus , clinically tested was GLS-5300, and was administered at doses of 0.67, 2, and 6 mg in three injections (weeks 0, 4, and 12). Vaccination in people aged 18 - 50 resulted in neutralizing antibodies which were lower than the antibody serum levels of patients in the acute phase of MERS. However, neutralizing antibody levels was similar to the level of antibodies in the serum of the recovered patients. Lastly, the vaccine did not evoke any side effects on people taking part in this trial (28).
3.2. mRNA Vaccines
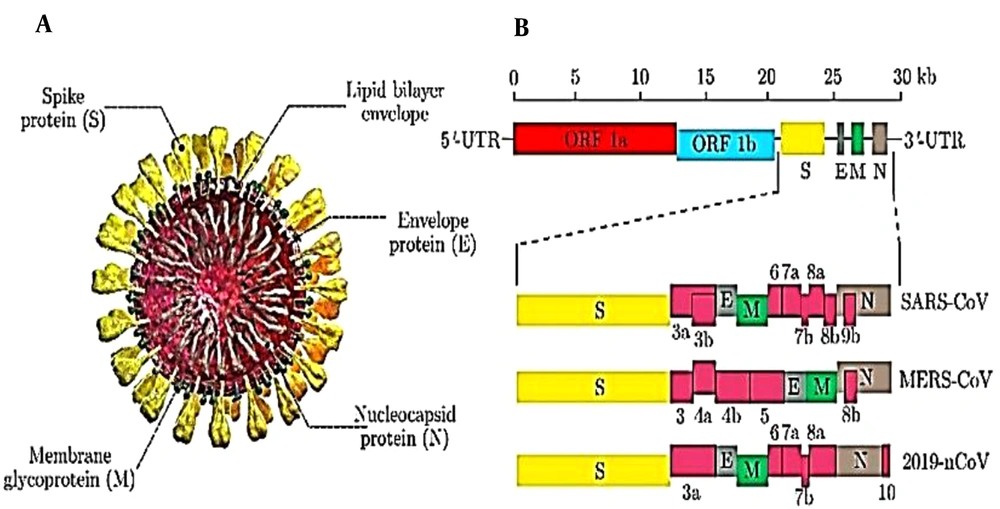
These vaccines have entered the clinical phase earlier than other vaccines due to their rapid production rate and development. Zeng et al. (2020) designed an mRNA vaccine containing COVID-19 virus antigens as well as appropriate 5/UTR and 3/UTR (Figure 1) (29).
Schematic structure of SARS-CoV, MERS-CoV, and 2019-nCoV. A, Schematic structure of virion of SARS-CoV, MERS-CoV, and 2019-nCoV along with its major structural proteins; B, Schematic diagram of genomic organization of SARS-CoV, MERS-CoV, and 2019-nCoV. The genomic regions or open-reading frames (ORFs) are compared. Structural proteins, including spike (S), envelope (E), membrane (M) and nucleocapsid (N) proteins, as well as non-structural proteins translated from ORF 1a and ORF 1b and accessory proteins, including 3a, 3b, 6, 7a, 7b, 8a, 8b, and 9b(for SARS-CoV), 3, 4a, 4b, 5, and 8b (for MERS-CoV), and 3a, 6, 7a, 7b, 8, and 10 (for 2019-nCoV) are indicated. 5/-UTR and 3/-UTR, untranslated regions at the N and C-terminal regions, respectively. Kb, kilobase pair.
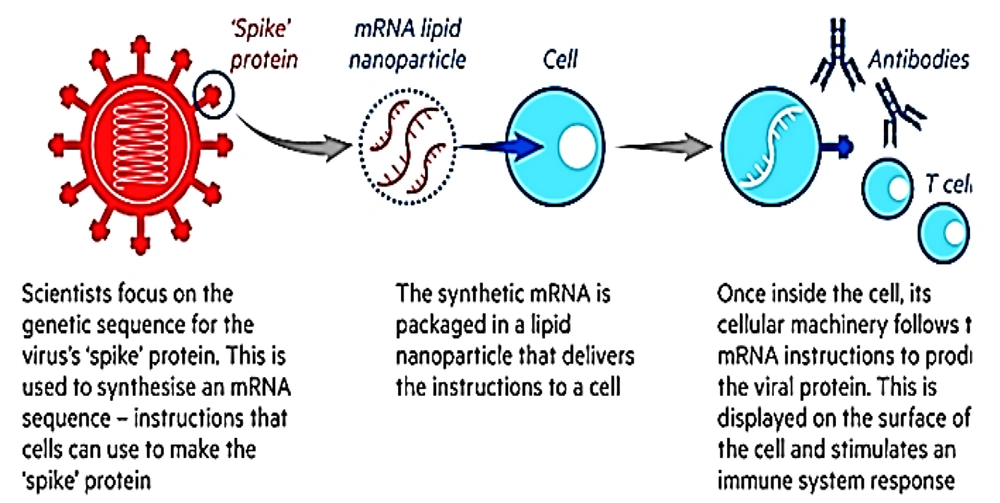
By selecting the appropriate 5/UTR and 3/UTR, Zeng increased the translation potential of this mRNA. They implemented TT3 particles to deliver the mRNA and reported that the application of TT3 nanoparticles instead of MC3 in mRNA delivery enhanced protein production (29). Pfizer (recently approved by FDA) and Moderna vaccines are considered as the main prototypes of mRNA vaccines, being encapsulated in lipid nanoparticles as a carrier (Figure 2). It should be noted that these vaccines are different in terms of RNA (BNT162b2 and mRNA-1273 for Pfizer and Moderna respectively) and lipid carriers (Table 1).
| Components | BioNTech/Pfizer | Moderna |
|---|---|---|
| Amino lipid | (4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis (ALC-3015) | SM-102 |
| PEG lipid | (2- hexyldecanoate),2-[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide (ALC-0159) | PEG-2000 DMG |
| Structural lipid | 1,2-distearoyl-snglycero-3-phosphocholine (DSPC) | DSPC |
| Cholestrol | cholesterol | Cholesterol |
Components of Lipid Carriers for BioNTech/Pfizer and Moderna
3.3. Vector-Based Vaccines
This group benefits from attenuated viruses such as MVA, adenovirus, and measles, whose safety has been approved in previous studies as a vector to deliver the nucleotide sequence encoding Coronavirus proteins (mostly spike protein). An ample example of this classification is ChAdOx1nCoV-19 known as Oxford vaccine (AstraZeneca). ChAdOx1 nCoV-19 is a chimpanzee (Ch) adenovirus-vectored vaccine (30). Accordingly, this vaccine contains the Coronavirus protein-producing genes in a harmless vector virus such as the common cold-replicated adenovirus (31).
Another approved vectored vaccine called Sputnik V (Gam-COVID-Vac), is an adenoviral-based vaccine against COVID-19. Sputnik V employs a debilitated virus to convey little parts of a pathogen and invoke an immune reaction. This vaccine comprises two human adenoviruses – a common cold virus – containing the gene that encodes the full-length spike protein (S) of COVID-19 to improve the immune response (32). Adenovirus is utilized as a “container” to deliver the Coronavirus gene to cells and initiate the synthesis of the new Coronavirus's envelope proteins, “introducing” the resistant system against it. The recombinant adenovirus 26 and 5 are both applied as vectors in Sputnik V. They are biotechnologically-derived and contain COVID-19 S protein cDNA. In addition, the Ad26-based immunization is utilized on the primary day and the Ad5 immunization is administered 21 days later to boost immunity (33).
The Johnson & Johnson COVID-19 vaccine comprises a replication-incompetent recombinant adenovirus 26 (Ad26) vector which expresses the COVID-19 spike (S) protein in a stabilized conformation (34).
In an interesting study, Munster and colleagues inserted a nucleotide sequence encoding full-length MERS-CoV spike glycoprotein in a kind of simian adenovirus portraying a replication defect. The vaccine is administered intranasally as well as intramuscularly. The results of this study reflected that the vaccine significantly increased the titers of neutralizing antibodies. Although the titer of neutralizing antibodies in the I.M. administration was higher compared to I.N. administration, this difference was not significant. What is more, this study analyzed the effectiveness of the developed vaccine for the prevention of MERS in hDPP-4 transgenic mice. Vaccinated mice were detected with no sign of MERS-CoV virus antigens in their lung tissues (34).
In another study, Kim et al. (35) reported that intranasal administration of a replication-deficient adenovirus expressing full-length S protein triggers T RM cells in airway and lung parenchyma (35).
Besides, Bodmer et al. (2018), expressed that although the UV-inactivated mv (measles virus) did not evoke immunity against MERS-CoV or mv, the attenuated mv virus expressing the S protein of MERS virus, induced multifunctional T cells immunity. Altogether, the designed vaccine was reported to be effective in older mice, regarding corona vaccination target group, it would be a piece of good news (36).
Furthermore, Li et al. (2020) compared a virus vector carrying MERS S1 protein with a bacterial vector (BLP particles) carrying the RBD segment of MERS Spike protein. In this study, the recombinant rabies virus expressing the S1 protein sequence of MERS virus (RV/MERS VIRUS) was implemented as a virus vector. The secretion of TNF-α, IFN-γ, and IL-2 cytokines was evaluated as a cellular immunity indicator as well as MERS-CoV RBD IgG, G1, G2a, G2b, G2c, G3 as humoral immunity indicator. The results indicated that the viral vector caused a quick induction of humoral immunity as well as a robust cellular immunity in comparison to the bacterial vector. Nonetheless, the bacterial vector (BLP) induced a higher level of neutralizing antibodies (37).
3.4. Live-Attenuated Vaccines
The application of this type of vaccine is limited due to the possibility of reversion and instability triggering the conversion to more virulent forms (38). In another study, TRS (transcription regulatory sequences) section of the SARS-CoV genome which is responsible for replication was rewired to achieve an attenuated virus without the risk of reversion. The results of this study revealed that the virus was stably attenuated together with the encouraging results in the prevention of the wild-type virus infection (39).
3.5. Inactivated Virus
A salient example existing within this category is known as BBIBP-CorV (Sinopharm COVID-19 immunization), one of the inactivated types of COVID-19 vaccines developed by Sinopharm Co, in China. Having that said, BBIBP-CorV offers comparable state-of-the-art technology compared with CoronaVac and BBV152, other inactivated virus vaccines for COVID-19, being developed in Phase III trials (39, 40).
In an interesting study, the SARS virus isolated from patients' blood samples was inactivated by formaldehyde and the obtained vaccine was injected into rhesus monkeys intramuscularly. The level of specific IgG increased from the seventh day of vaccination in a dose-dependent manner. The level of induced nAbs was close to those of DNA vaccines as well as inactivated-virus vaccines but higher than nAbs induced by adenoviral-based vaccines in monkeys.
In this study, the level of IgA, a mucosal immunity indicator significantly increased. Moreover, considering the enhancement of IFN-γ level in the serum of the vaccinated group, the immune response evoked by this vaccine is a Th1-bias response. What is more, the results of viral challenge on rhesus monkeys exhibited that the control group displays symptoms of low-grade fever, viremia, and viral replication in the lungs while all 4 vaccinated monkeys with high doses of the vaccine (50 ug) were completely protected against the viral infection. This protection against other strains of the virus was observed, highlighting the cross-protection feature of this vaccine (40). Spruth et al. in another study inactivated the SARS-CoV by formaldehyde and subsequently UV-radiation to ensure virus inactivation. The vaccine was injected subcutaneously and the results demonstrated that it appeared to be perfectly efficient at inducing high antibody titers against Coronavirus S protein. Besides, it also increased the level of IFN-γ as well as IL-4 which are indicators of Th1-bias and Th2-bias immune response, respectively. In this study, Al (OH)3 was used as an adjuvant that its effect on enhancing the immunogenicity of the vaccine was inappreciable. Finally, the vaccine also prevented the SARS virus replication in the respiratory tract of CD1 mice regarding the viral challenge (39).
3.6. Recombinant Protein Vaccines
McPherson et al. (41) have tried to explain SARS spike protein immunogenicity evaluation methods in Vaccine Design: Methods and Protocols, Chapter 14th. In this book, they established a recombinant SARS S ∆TM protein which does not have the interstitial and cytoplasmic part of this protein, however, it contains the RBD part as an immunogenic motif. They have also used the baculovirus/insect cell system as recombinant protein. Due to the low immunogenicity of subunit present in the vaccines, the application of adjuvant is highly recommended in these vaccines. Since aluminum-based adjuvants have driven the immune response into the Th2 biased immune response triggering eosinophilic immunopathy in lung tissues, McPherson declares that the application of delta inulin in this type of vaccine is more appropriate than aluminum-based adjuvants. Nevertheless, delta Inulin-based adjuvants promote Th1 and Th2 immune responses in a balanced trend. It was also recommended to employ balb/c mice in vaccine immunization evaluation as the vaccine’s preventive effect on infection depends on the serum level of neutralizing antibodies and that they trigger a Th2 immune-bias response (41).
In another study, a recombinant SARS S ∆TM protein was developed by Zhou et al. according to the method recommended in McPherson's book and then evaluated the immunological effect. The results indicated that this vaccine can significantly increase neutralizing antibody titer in mice. Furthermore, aluminum hydroxide was employed in the formulation as an adjuvant and no difference considering the immune response was observed between the adjuvant-receiving group and the group not receiving the adjuvant (42).
3.7. Employing Microneedle Arrays (Mnas) in Recombinant Subunit Vaccines
According to the previous studies, spike protein of Coronavirus present on the surface of this virus are present trimerically, unlike recombinant proteins usually bearing a monomeric structure. To produce the spike protein in trimeric form, Kim et al. designed a DNA sequence, encoding the S1 segment of MERS-CoV and COVID-19spike protein as well as RS09 and flagellin as adjuvants, and T4 fibritin folds on trimerization domain at the end of DNA sequence to develop a trimeric form. Later, they placed this DNA sequence in an adenovirus vector to infect cells and to trigger protein production. In this study, MNAs were applied to deliver the recombinant proteins and the results revealed that this vaccine evokes a long-lasting higher titer of virus protein-specific IgG as well as inducing stronger antibody-mediated neutralizing activity compared with s.c administration. The effect of these two applied adjuvants in immunization amplification was minimal in delivery by MNAs, while in s.c administration the flagellin had no considerable effect and RS09 displayed a negligible effect. The vaccine also protected hDPP4 transgenic mice against MERS-CoV infection in the viral challenge (43).
3.8. Nanoparticles in Coronavirus Vaccine
McKay et al. (44) encapsulated a saRNA encoding COVID-19 spike protein in lipid nanoparticles and the particles were injected into balb/c mice, at one-month intervals two times. To stabilize the pre-fusion conformation of the S protein, lysine 968 and valine 969 of this protein were substituted by proline. Next, the serum of vaccinated mice was collected and exposed to COVID-19 to investigate the neutralizing effect of vaccine-induced antibodies. Finally, the results indicated that the serum had a high neutralizing effect in a dose-dependent manner which was higher than the recovered patients’ serum neutralizing effect, therefore, a positive correlation was observed between viral neutralization and serum level of antibodies.
To evaluate the cross-reactivity of induced antibodies, the serums were exposed to SARS-CoV, MERS-CoV, and 229E-CoV pseudo-types of Coronaviruses, which demonstrated a negligible neutralizing effect. Besides, concerning the cellular immunity, splenocytes of vaccinated mice re-stimulated by COVID-19 peptides and IFN-γ-secreting T cells were determined by ELISpot which was significantly higher than control groups in a dose-dependent manner.
It is worth noting that EP DNA was used as positive control and saRNA, which encodes the glycoprotein of rabies in pABOL, was implemented as the negative control (44). Moreover, Coleman and colleagues exploited the self-assembling property of an amphiphilic protein aggregate to fabricate nanoparticles comprising Coronavirus spike protein. In this study, S protein trimmers formed 25nm-sized micelles. The particles were injected into balb/c mice intramuscularly accompanied by either aluminum hydroxide or Matrix M1 as an adjuvant. The results of the study illustrated that without the application of adjuvant, this protein could not elicit an immune response efficiently. Furthermore, the Matrix M1 Adjuvant is significantly more effective than aluminum hydroxide in terms of increasing the quantity of nAbs. Regarding Matrix M1, there was no considerable difference between the 1ug and 3 ug doses of the vaccine, indicating that lower doses of S protein could be administered in the presence of Matrix M1. In addition, there was no difference in the amount of neutralizing antibodies on the 21st day or 45th day after vaccination in none of the vaccinated groups underlining the fact that nAbs did not change during the vaccination course (45).
Negahdaripour et al. (46) investigated microneedle array (MNA) as a means to deliver trimeric recombinant subunit vaccine against S1 protein of MERS-COV-2. They found that using dissolvable MNA for delivery of MERS-COV-2 can induce powerful humoral response with or without adjuvant (flagellin or RS09, as TLR5 or TLR4 agonists, respectively) in comparison with subcutaneous injection. Therefore, by using dissolvable MNA, long-lasting and potent antigen-specific immunity response beginning 2 weeks after immunization was induced.
The application of MNA for vaccine delivery has potential benefits including high density of antigen presenting cells and different immune accessory cells in skin environment, Low dose antigens for inducing a robust immune response by reducing production costs and required dose, Self-administration of vaccine as well as the stability of vaccine component in MNA polymer matrix (43).
4. Nonspecific Vaccines
4.1. BCG Vaccination
The Bacille Calmette-Guerin (BCG) is a live attenuated vaccine consisted of the bacteria causing bovine tuberculosis (Mycobacterium bovis). It was produced approximately 100 years ago and when administered under the skin (intradermally) to a newborn child, it protects them from the severe and disseminating disease manifestations of human tuberculosis (caused by Mycobacterium tuberculosis infection). Amazingly, BCG vaccination seems to not only protect children against severe tuberculosis, but offers non-specific protective effects against other respiratory tract infections approved by in-vitro and in-vivo studies, and thus this vaccine is being repurposed to ensure if it can reduce morbidity and mortality rates associated with COVID-19 infection (47-50).
Numerous studies have been undertaken to discover a link between BCG vaccination and susceptibility to COVID-19 infection. Previous studies have shown BCG vaccine, a weakened type of Mycobacterium bovis, can increase CD4+ cells, IFN-γ, and interleukin-3 (47). In addition, the vaccine reduced infant mortality, independent of reducing the incidence of tuberculosis (51).
Among several studies, examining the hypothesis of BCG vaccination on decreasing COVID-19 was a critical debate, some have supported this hypothesis (47, 50, 52, 53) meanwhile others rejected it (54, 55).
Miller et al. (2020) reported that along with the BCG vaccination plan, the starting time of universal BCG policy also imparts a critical role in the intensity of COVID-19 disease in different countries. Countries such as the United States, Italy, and the Netherlands that have never had a public vaccination program and BCG vaccination was limited to high-risk groups, experienced a large number of severe conditions considering COVID-19 epidemic. However, countries such as Iran have experienced a high mortality rate despite the public vaccination policy as the starting time of universal BCG policy was late (1984) having their elderly population not being vaccinated (50).
In a comprehensive study, Shet et al. (2020) examined the effect of BCG vaccination on COVID-19 mortality. In this study, they tried to eliminate other factors affecting the mortality rate of COVID-19. Therefore, to overcome the pervasive challenge of differential epidemic time on the mortality rate of this disease, the time of examination is considered since the observation time of the 100th case of infection in each country. To eradicate the age average and economic status of countries, they categorized the studied countries in terms of per capita GDP and population over 65 years of age. Statistics showed that COVID-19 mortality in low-income countries that typically have a younger population has the lowest rate (51).
In another study, Hines investigated the effect of BCG vaccination policy on the mortality rate of COVID-19 by employing a simple linear regression model. Correspondingly, countries were divided into three groups based on the vaccination policy: current national BCG vaccination policy, past national BCG vaccination policy, and vaccination of specific groups or none. Consequently, it was observed that the COVID-19 mortality rate was lower in countries with the current national BCG vaccination policy compared to the other policies (52).
Dayal and Gupta prepared a brief report and declared that among countries with high COVID-19 restrictions including China, Italy, the United States, Iran, Spain, the United Kingdom, South Korea, Germany and France, the case fatality rates (CFR) of COVID-19 was lower in countries executing universal BCG vaccination policy (56). Akiyama and Ishida analyzed the effect of BCG vaccination on COVID-19 mortality in groups of 30 years of age. Moreover, they applied death doubling time (DT) instead of CFR to minimize the effect of the epidemic stage on mortality. The final results revealed that DT in the vaccinated group was higher than the unvaccinated group. Furthermore, they reported that DT in countries using Tokyo 172-1 strain was lower than the receivers of other strains (53).
All of these studies have approved the effect of BCG vaccination on reducing COVID-19 mortality and morbidity although it is suggested to categorize countries in terms of their BCG vaccination coverage instead of BCG vaccination policy.
Contrary to the aforementioned studies which are in agreement with BCG vaccination effect on COVID-19 incidence reduction, another study indicated that countries with less than 89% BCG vaccination coverage (such as Finland, Sweden, South Africa, Greenland, Iceland, Iran) did seem to have a higher corona-related fatality. The study found that deaths from COVID-19 were more correlated with tuberculosis incidence than with BCG vaccination (negative correlation), therefore, the author examined LTBI (latent TB infection) and concluded that countries with higher LTBI have lower CFR. Moreover, it was reported that this correlation does not prove any effects of LTBI on COVID-19 as countries with a high burden of the disease have high economic relationships and tourist exchanges with China (55). Another study came to a completely contradictory result considering other studies in the effect of BCG vaccination on reducing COVID-19 incidence and mortality. The author expressed that although protestants avoided any vaccination due to their religious beliefs in the Netherlands, the disease reflected a lower incidence in protestants than catholic.
Besides, Denmark with 63 percent protestant individuals, was reported to have fewer COVID-19 cases in comparison to other countries. These results were completely inconsistent with previous results concerning BCG vaccination on reducing COVID-19 mortality. The low incidence of disease in protestants was attributed to the prohibition of participation in carnivals, thus it is necessary to consider the socio-structural together with cultural factors in such studies (55).
Hensel et al. (2020) confirmed a lower incidence of COVID-19 in countries with current universal BCG vaccination policy even though the policies of these countries were not different and the countries that never had a universal BCG policy or those countries with a BCG vaccination policy in the past (54).
Even if this hypothesis is correct, will vaccination of the elderly, who are among the most vulnerable people, protect them against COVID-19? Shet et al. reported that three times of injections during a month significantly reduced upper respiratory infections in people over 65 years of age as well as viremia in adults. Given this fact and considering that the BCG vaccine has been administered more and bears safer profile among other vaccines (51), it makes sense to design a clinical trial to evaluate the efficacy of this vaccine concerning the current COVID-19 crisis. Last but not least, it is recommended to consider other factors such as vaccine brand, virus strain used in a vaccine, etc. in future studies.
4.2. MMR (Measles, Mumps, and Rubella) Vaccination
Measles, mumps, and rubella are all viral diseases that can lead to serious consequences. The measles-mumps-rubella (MMR) vaccine is comprised of live-attenuated strains of these viruses, considered as a safe and effective way to prevent these diseases, especially in young children. Interestingly, young children do not seem to be overly susceptible to COVID-19 while they are infected by other diseases. One hypothesis to elaborate on this phenomenon is related to the formed antibodies against measles (due to the MMR vaccine) acting as cross-reactive agents concerning SARS-CoV-2 (57). Thus, MMR vaccination may be an effective action against COVID-19. The homology between amino acid sequences of COVID-19 macrodomain and rubella virus has led to the design of a study to investigate the effect of MMR vaccination on COVID-19 incidence and mortality. In this regard, Young et al. designed a study to analyze MMR vaccination protection against COVID-19. In this study, MMR vaccination policies in three countries with high COVID-19 burden, namely, Germany, Italy and Spain were explored. The study reported that age groups without MMR vaccination coverage had the lowest levels of immunity against COVID-19. Besides, the macrodomain of the new Coronavirus could be detected by antibodies generated against rubella virus. In COVID-19-infected cases, rubella specific IgG level increased as much as a secondary rubella infection. Ultimately, the results of this study indicated that although MMR vaccination may not prevent COVID-19 infection, it can improve the immunity of individuals against the disease. To ensure the accuracy of this hypothesis, it is necessary to design a study to prepare individual-based data in the targeted population (57).
4.3. Oral Polio Vaccine
This is a member of inactivated virus vaccines and its oral form contains an attenuated strain of the virus. Random mutations and attenuation occur as a result of production by non-human cells under sub-physiological temperatures. Ongoing studies have demonstrated non-specific effects along with reduced morbidity/mortality statistics regarding non-polio infections. Later, this vaccine was tested to determine whether these effects could be identified in COVID-19 infection cases (58).
4.4. IMM-101
This is a heat-inactivated vaccine, known as a chemotherapeutic agent and has demonstrated immune memory effects that may be attributed to non-specific antiviral impacts. Thermal processing imposes a lower risk compared to attenuated or live vaccines (59).
4.5. AlloStim
This is a bioengineered living-cell vaccine containing SARS-CoV-2-specific modified cells from healthy donors which elicit both memory and effector cell functions. The vaccine stimulates an immune response as soon as detecting the native virus, triggering rapid viral clearance and viral-specific memory (60).
4.6. BACMUNE (MV130)
This vaccine is a polyvalent and heat-inactivated Gram-positive-negative bacteria that is administered orally. It activates the immune system which is practical impressive in other infections such as SARS-CoV-2 concerning a non-specific manner (https://biorender.com/covid-vaccine-tracker).
4.7. V-SARS
The V-SARS vaccine is made of the heat-inactivated plasma of COVID-19 donors. Engineers contend that individuals infected with COVID-19 develop a circulating COVID-19 virus, and heat-inactivation process of their plasma delivers an inert virus to healthy people and evokes an immune reaction against this pathogen (https://biorender.com/covid-vaccine-tracker).
4.8. RUTI
This vaccine is currently being tested as a tuberculosis vaccine candidate. It is predicted that it induces a positive effect on other infections following a non-specific manner. It thus may be beneficial in preventing infections like SARS-CoV-2 (https://biorender.com/covid-vaccine-tracker).
Considering the registered clinical trials reviewed above, the immunity level of BCG vaccine is higher than other candidates present in the market, in addition, it is too early to comment on the fate of the developing vaccines. More studies should be undertaken to make rational decisions regarding this issue.
5. Considerations and Safety of COVID-19 Vaccines
While the immunity induced by the vaccines can be protective against SARS-CoV-2, vaccines could impose negative impacts on infected tissues, too. In this regard, there are two limitations concerning the safety of developing vaccines: cellular immunopathology and antibody-dependent enhancement (ADE) (61). To evaluate the vaccine efficacy from this point of view, it is required to determine a detectable factor as an indicator for ADE as well as cellular immunopathology. In the absence of a reliable marker for evaluating these phenomena in animal experiments, some of the aforementioned studies are reviewed to discuss several factors concerning ADE and cellular immunopathology to adopt a rational approach to design studies related to vaccine development (38).
Since immunopathologic events depend on many factors such as the applied adjuvant, vaccine delivery platform (61), and vaccine formulation regarding the particle size, it is suggested that in addition to selecting an appropriate vaccination platform, a suitable adjuvant as well as formulating a proper particle size should be taken into account.
Regarding vector-based vaccines, although the immunogenicity level of these vaccines is lower than other models, due to the long-term administration of these vaccines, there is no concern about unknown side effects. In general, the advantages of viral vectored-based vaccines including the desired level of immunogenicity, not being neutralized by the immune system before immunization against the disease. One of the major concerns about these vaccines is associated with the possibility of integrating viral vectors into the human genome (62).
In the case of the attenuated virus-based vaccine, one of the advantages of this vaccination mode is sufficient immunization in most people upon receiving the same first dose. The main drawback of these vaccines is the probability to become a common pathogenic virus in body (few cases have been reported). Moreover, this type of vaccine cannot be prescribed to people with defective immune systems (people with AIDS). Nonetheless, inactivated virus-based vaccines are less risky. In these vaccines, the virus is inactivated by heat or a chemical agent after being cultured in the medium. The main advantage of inactivated vaccines is related to their application in people with weakened immune systems. However, these types of vaccines require multiple doses to develop the intended level of immunity (63).
In a protein-based inactivated vaccine there is not any virus, instead, an immune-generating antigen is developed within a biological environment (such as hepatitis B vaccine). In these types of vaccines, the selective antigen is of utmost importance, and if the selected antigen fails to adequately stimulate immunization, the developed immunization will be weak and temporary (62).
Despite the rich research evidence considering the treatment of diseases such as cancer and rabies, genetic or nucleic acid (DNA, RNA) based vaccines are being applied for the first time globally. Due to the novelty of these models, the need for risk assessment is essential.
6. Challenges of COVID-19 Vaccines
Identification of antigenic epitopes is the most important phase in vaccine design. So far, spike protein (S) of COVID-19 has been identified as a promising target vaccine development, while choosing the receptor-binding domain (RBD) or the full-length protein remains a challenging question (62, 63).
Another challenge is referred to the time-consuming process for evaluating the efficacy and safety aspects of vaccines. Although bioinformatics detects epitopes quickly and accurately compared to the conventional methods, the limitation of time-consuming clinical phases remains a challenge in emergencies (62-65).
Traditional or bioinformatics techniques do not deliver sufficient genomic data when a new epidemy is developed by a new virus or a new strain of an old virus. Hence, dry and wet labs are great candidates for foolproof vaccine development.
Basically, conventional vaccine production methods use cultivation of pathogens and further identify their immunogenic components. This method is time-consuming and another limitation would be highly produced amount of antigens. In addition, sometimes the antigens are produced during the disease while their production is not possible in the laboratory. On the other hand, these methods cannot be applied to non-cultivable microorganisms (66, 67).
Since bioinformatics is still in the infant period, there are uncertainties in genomic and proteomic, leading to some challenges in the detection of T-cell and B-cell epitopes. To illustrate, there are still a significantly large number of genes and genomic sequences where one or more of the constituents have remained ambiguous. On the other hand, antibody-antigen interactions are significantly dependent on antibody structures and differences in amino acids (even one of them) which could inhibit their binding process. Thus, false or defective bioinformatic data result in the design of ineffective vaccines. Moreover, bioinformatics is considered as a safe and simple strategy without any adverse effects, especially in subunit vaccine design. Nevertheless, it suffers low immunogenicity that may be addressed by appropriate adjuvants, immunization schedules, and suitable antigenic epitope selection (68).
The limitations for RV include (1) identification protein antigens (only) while polysaccharides and glycolipids can also be proper candidates for vaccine development; (2) efficacy for prokaryotic organisms owing to the complexity of Eukaryotes genome (68).
On the contrary, sequential mutations in the virus genome hinder the development process of effective vaccines. Therefore, developing a broadly protective vaccine against the whole virus family is critical to overcoming this pervasive challenge (69). What is more, concerning the identification of high-risk populations, specific serologic and clinical studies must be undertaken (70).
Immunogenicity, genomic structure, pathogenicity, epidemiology, target antigen, correlated immune protection, outbreak forecasting, target population cellular and humoral immune response against the virus are considered as essential information for the design and development of COVID-19 vaccines (71-73) and despite tremendous endeavor of the scientific community, there are many undiscovered facts and unanswered questions.
Local reactions such as swelling, redness, and pain at the injection site together with adverse systemic reactions such as body aches, fever, and chills are detected following the administration of COVID-19 vaccines. These effects can be attributed to the adjuvant while it is necessary for evoking robust immune responses in combination with antigenic epitopes (73).
The most challenging fact concerning live attenuated vaccines is defective inactivation of viruses triggering ADE responses which should be evaluated by precise quality control methods. However, these vaccines can bring about a strong immune response if they are organized perfectly.
Considering vector-based vaccines, anti-vector immunity can be considered as a challenge while these vaccines can induce neutralizing antibody and cellular immune responses (74).
Although Nanoparticle and DNA-based vaccines endow a safe profile, lower immunogenicity of these vaccines in comparison with the others, requirement for an appropriate adjuvant, immunization type, and optimization of antigenic epitope sequence for inducing boosted immune response are still some grave limitations in this approach (74).
While administering existing vaccines e.g. live-attenuated or inactivated vaccines with sufficient c-GMP is safe, novel mRNA, DNA, and RNA vaccines do not have the same protocols and there is a need to design it which is time-consuming (75).
Pfizer seems to have overcome the toughest technical hurdles (FDA approve). The company has developed a vaccine that appears to be 95% effective in preventing COVID-19. However, this is only a temporary analysis and a preliminary investigation along with the side effects and consequences for some recipients have raised doubts considering the company's vaccine. The COVID-19 mRNA vaccines are the first in this group to be marketed and tested on humans. Hence, no one can determine its long-term effects, but it can be assumed that due to the possible mutations, this vaccine has a long-term effect and generates a lot of antigens. According to the manufacturer, the vaccine may cause dysregulation in the immune system and later develop a large antigen-antibody complex, triggering deposition and disruption of other tissues. Besides, if the secondary structure of the mRNA itself changes in response to a new antigen, antibodies will be generated against it (warning on genetic manipulation and the risk of autoimmune diseases and cancer in the coming years), however, these must be experienced over years to ensure the efficacy. The application of the messenger RNA (mRNA) is highly unstable, and no mRNA vaccine has yet been approved for human administration. Production of mRNA is carried out in specially designed stainless-steel bioreactors which requires sterile conditions together with stable temperature and humidity. Instability is one of the reasons why it is necessary to keep this vaccine cold at very low temperatures (76). The Pfizer vaccine should be stored at -70°C (94°F). The freezers storing this vaccine at -70°C are not cheap nor abundantly available. The vaccine could be blended with a sterile fluid - usually water - at the injection site and injected within six hours of preparation. Because the vaccine is sometimes given at high doses, rural communities may not have the population or infrastructure to inject doses of the vaccine while they are still cold. For this reason, residents of rural areas who want to be vaccinated may need to travel to nearby cities. Another limitation is accessibility of poor countries to vaccines. Most countries are not capable of producing the mRNA vaccine as well as providing cold storage conditions. If these countries want to offer the Pfizer vaccine to their citizens, they must find a way to deliver the vaccine to their territory in less than 10 days from the United States and inject it to citizens. The same problems exist for Moderna vaccine.
Considering AstraZeneca vaccine, blood clot formation and low platelets count with bleeding (sometimes) have been raised as serious problems. On the other hand, this vaccine has been tested in individuals under 65 years old as scientists were hesitant for older ones. German scholars have raised an immune reaction in which antibodies trigger platelet to form clots. Such reaction has already been observed by thinner blood caused by heparin. This subject is very important and therefore they published a description as vaccine-induced prothrombotic immune thrombocytopenia (VIPIT) (77). This claim was partially refuted by the manufacturer and the World Health Organization. Despite the growing evidence of clot formation and the denial of the manufacturer, the judgment will not be simple.
According to the WHO reports, the most important side effect of Janssen vaccine is Flu-like symptoms (besides injection site pain, headache). However, generally, the side effects of this vaccine are lower than Pfizer and Moderna especially in comparison to their second dose (Janssen is a single dose vaccine).
7. Advantages of Vaccine Platforms
Bioinformatics provides a valid and powerful tool in identifying epitope regions with the aid of robust computational equipment. Traditional vaccine development methods are time-consuming and are not useful in rapid outbreaks and to be employed for large populations. Generally, the advantages of in-silico epitope-based vaccines are rapid and accurate design, time/cost-effective formulations, and desired immunogenicity with minimized adverse effects (78).
RV can identify new antigens (besides all identified antigens by conventional methods) that play an important role in the immunogenicity of new generation vaccines while these antigens were not detected previously (79).
Bioinformatics aid researchers to solve problems which cannot be addressed by laboratory methods, indeed, bioinformatics offers the chance to find functional information which has a profound effect on molecular immunology. Likewise, Bioinformatics helps to reduce the number of possible peptides and faster vaccine development in comparison to conventional methods (80).
Subunit vaccines including S1 protein and RBD domain of COVID-19 are worthwhile targets and induce a stronger immune response than the whole virus particles (inactivated or attenuated viral vaccines) then development of these vaccines provide efficient prevention (81).
DNA vaccines have demonstrated lesser typical syndromes in animal studies such as pneumonia. mRNA vaccines reflect several advantages over conventional forms: low-cost production, safe administration, high potency, and short production process (82).
Combining different peptides into a vaccine (called multivalent vaccines) is another advantage of peptide-based vaccines in comparison with whole protein vaccines (traditional) to enable as wide coverage as possible thus that one or other epitopes will elicit an effective immune response.
Production of oral or nasal vaccines is suggested since immunization routes such as oral or aerosol which induce mucosal immune responses are the optimal approaches to reach a proper immune response (35).
8. Future Prospect
Will the corona vaccine end the corona crisis?
Experts have discussed the end of the corona epidemic based on three main factors including lack of corona vaccine, vaccine effectiveness and, genetic mutations.
Currently, one of the biggest and most important challenges is providing adequate vaccines for the vaccination process in different countries. The inability of vaccine companies such as Pfizer, Moderna and AStraZeneka to supply the vaccine has prompted the European Union to threaten to limit the export of domestically produced vaccines to other countries to make up for the shortage of corona vaccines. Thus, the vaccine shortages is the biggest obstacle to tackling the global corona epidemic crisis, at least in the short term (63, 64).
Another challenge is the effectiveness of different types of vaccines. Pfizer has announced that its vaccine is more than 90% effective, while this is 94.5% for Moderna seven weeks after receiving the second dose, and almost 70% for Johnson & Johnson. According to experts, effectiveness of more than 60% can control the COVID-19 epidemic. However, due to the different percentages of immunization concerning different vaccines, the degree of immunization will also be different in different societies, and in this regard, in near future, different communities may have different positions in terms of immunization against corona (64).
Genetic mutations are the third factor in determining the future of corona. Even if the products required for the corona vaccine are supplied worldwide and the effectiveness of different corona vaccines is at its highest, an uncontrollable issue in the face of the corona epidemic is the genetic mutations in the virus. So far, two types of corona mutations, including British corona mutant and South African mutant corona, have attracted a great deal of attention, and it has been proven that the rate of outbreak and epidemic of the British corona is many times faster than the previous types. The vaccine response to these genetic mutations varies, for example, while the Oxford Corona vaccine has promising effects against British mutant virus, the same vaccine did not have the expected efficacy against the mutated South African virus (65).
On the other hand, it is not clear how long these corona mutations will continue and to what extent of existing vaccines will be able to resist against corona genetic mutations, but alarms are already ringing over possible and unpredictable mutations regarding the corona virus and the possibility of reducing the effects of existing vaccines (66).
Some evidence has demonstrated that utilizing BCG vaccine could boost the immune response against viral infection but more research should be conducted to prove the efficacy against CVID-19 (67-69). In contrast to previous findings, recent studies have indicated that the low fatality rate is not attributed to BCG vaccination (70). MMR vaccine might be capable of protecting against COVID-19, and is considered safe and time-saving for the elderly. However, more studies should be undertaken to support this idea that MMR vaccines may be effective enough to prevent COVID-19 infection or not (57).
Using nanomaterials for the development of vaccines can be a promising tool to combat COVID-19 pandemic. mRNA-lipid-based nanoparticles against SARS and MERS are currently being evaluated and technology will be applied to COVID-19, yet, Vectored-based and inactivated viruses are in the next steps (56). Moreover, nasal and drop formulations are being developed that have displayed interesting results in clinical trials.
According to the success of Pfizer and Moderna vaccines as two primitive alternatives, there are more trusts within the future for final vaccine improvement and treatment of COVID-19. The progress of COVID-19 vaccines is fast because they were fabricated by applying an unused platform known as gene-based technology which restrains mRNA to basically instruct the human body to develop the vaccine itself. By contrast, Customary vaccines that utilize either a debilitated virus or filtered signature viral proteins to incite the body to safely evoke immunity (that's compelling, but the act of developing the weakened virus or purifying the proteins is slow and difficult) mRNA vaccines, can be developed nearly as rapidly as a virus can be genetically sequenced. It can be considered as a new period for vaccines and vaccinology (65, 83).
It is anticipated that mRNA innovation will make significant strides in terms of producing modern vaccines which would be tremendously advantageous for other possible pandemic hits in future. COVID-19 vaccine has proven technological advancement at its best according to the significant steps and investigations explained in this revision.
9. Conclusion
In the present revision, numerous technologies, used to develop COVID-19 vaccines are explored in detail. Concerning the critical developed circumstances due to the widespread of COVID-19 including high mortality rate, disruption of life and, economic hardships, the distribution and application of vaccines have captured the attention of communities. Nonetheless, accurate information regarding the pros and cons of vaccines is not currently available. Hence, it is difficult to determine which vaccine is the most optimal in terms of preventing COVID-19 (taking into account the prevention level, side effects as well as the balance between them). Many studies have demonstrated that modern technologies such as bioinformatics or the development of mRNA-based vaccines have reflected promising results considering emerging pandemics. Accelerating the identification of genomes and epitopes of microorganisms and then providing a tool for the rapid and efficient design of vaccines are among the advantages of these methods. To sum up, mRNA vaccines being manufactured for COVID-19 virus is a promising technology which could be applied to develop different types of vaccines for other diseases.