Introduction
Coronary artery bypass grafting (CABG) surgery is a treatment of choice for many patients with coronary artery disease (1). Patients undergoing CABG surgery are at risk of serious complications (2) , such as acute kidney injury (AKI), with an incidence rate ranging between 5 - 42% (2, 3). AKI causes an increase in-hospital mortality by 10–30%, which can be increased even by 40–60% if dialysis is required (4). Many factors have been suggested in preventing AKI after cardiac surgery, including minimizing the usage of ischemic and nephrotoxic agents, decreasing renal vascular resistance, and inducing alkalinization of tubular fluid (5, 6).
In this regard, several studies have investigated the effect of pharmacological interventions to prevent AKI, such as N-acetylcysteine, mannitol, vitamin E, steroid, statin, dopamine, fenoldopam, and sodium bicarbonate. However, many of these preventive strategies failed to decrease the incidence rate of AKI (7-12), or the findings of these studies might not be very reliable because of the minor or poor quality of these studies (13, 14). Therefore, there is an increasing interest in decreasing post-operative AKI in order to improve clinical outcomes. There is a large body of growing evidence showing that acetazolamide can play a role in preventing AKI (15-18). Various mechanisms of acetazolamide, including alkalinizing renal tubular fluid (17), scavenging reactive oxygen species (16), and improving renal circulation (18) have been investigated in the previous studies. However, the role of perioperative administration of acetazolamide is unclear in kidney function after surgery. Moreover, the previous studies are heterogeneous regarding doses and acetazolamide administration, length of follow-up, outcome ascertainment methods, and other characteristics in the studied human subjects or animal models that have led to inconsistent results. Therefore, the present study was conducted to evaluate the effects of acetazolamide on AKI prevention after CABG surgery.
Experimental
This study was approved by the Ethics Committee of the Shahid Beheshti University of Medical Sciences, Tehran, Iran (Ethics code: IR.SBMU.PHARMACY.REC.1399.146) with registry code of IRCT20151227025726N21 in the Iranian registry of clinical trials (IRCT). Informed written consent was obtained from all the patients before enrollment in the study.
The present randomized, open-labeled clinical trial was conducted in the Shahid Modarres Hospital—a tertiary university hospital in Tehran, Iran, on the patients who were candidates to undergo the CABG surgery from January 21, 2020, to February 8, 2021. The sample size was calculated based on 80% of power and 95% of confidence level; and significance level (α) was assumed to be 0.05. The highest number was selected for sample size.
All the patients aged more than 18 years old and admitted to the hospital to undergo elective on-pump CABG surgery were enrolled in the study.
Exclusion criteria were patients with 1) a history of hypersensitivity to acetazolamide, and any sulfonamide compounds; 2) stages IV and V of chronic kidney disease based on the modified diet in renal disease (MDRD) equation; 3) liver failure (Child-Pugh stages B and C); 4) left ventricular ejection fraction (LVEF) lower than 30 %; 5) hypokalemia (potassium <3.3 mmol/ L); 6) hyponatremia (sodium < 135 mmol/ L); 7) gout attacks; 8) metabolic acidosis; 9) and the need for undergoing emergency CABG surgery.
An online statistical computing web program (www. Sealedenvelope.com) was utilized to randomize the assignment of the patients in acetazolamide or control groups. The patients in acetazolamide group received 500 mg acetazolamide tablet (Darou Pakhsh, Iran) 2 h before surgery, while the control group did not receive acetazolamide.
The patients’ demographic data, medical and drug history, as well as laboratory data were also documented.
The basic kidney function was monitored before and after CABG surgery. Serum creatinine concentration (SCr) was usually measured daily using Jaffe chemistry techniques with a total imprecision of < 6%. Every patient’s creatinine value was analyzed. Both relative and absolute increases in SCr concentration were used to diagnose and classify AKI stages according to the kidney disease-improving global outcomes (KDIGO) criteria; daily urine output and the need for dialysis were also recorded (Table 1).
Induction and maintenance of anesthesia, and surgery were conducted using the same method in both groups. All the patients in both groups received the same standard surgery protocol of intravenous fluid type based on their weight, utilizing ringer, albumin, heparin, corticosteroid, diuretic, and cefazolin as preoperative antibiotic prophylaxis, which is designed for patients underwent on-pump cardiopulmonary bypass surgery according to the the patient’s hemodynamic status. One cardiac surgeon performed all the CABG surgeries according to the standard practice guidelines. Perioperative data, such as anesthesia time, cross-clamping time, cardiopulmonary bypass time, intubation time, need for intravenous fluid (IV), inotropes, blood transfusion, and nephrotoxic drug were also recorded. All the patients were transferred to the intensive care unit (ICU) according to standard protocol. C-reactive protein (CRP) level and LVEF were measured in all the patients at baseline (on morning before surgery) and 24 and 48 h after surgery, respectively. Levels of CRP were measured using the CRP-latex immunoturbidometric assay (CRP-LIA).
Adverse reactions regarding the use of acetazolamide and surgery complications were evaluated and described in each group based on the Naranjo scale.
Patients were followed up for 7 days to evaluate AKI after CABG surgery based on KDIGO criteria as a primary outcome (19). All the patients were followed up until discharge.
Secondary outcomes, duration of post-operative mechanical ventilation, ICU, hospital length of stay need for dialysis, and mortality rate were evaluated.
Statistical Analysis
All the statistical analyses were performed using SPSS software for Windows (Version 23.0; SPSS Inc., Chicago, IL, USA). Categorical and nominal variables were expressed as frequency (%) and were compared using the Chi-Square test. Continuous variables were tested for normal distribution by the Kolmogorov–Smirnov test. Data were expressed as means/standard deviations or median, interquartile ranges (25th and 75th percentile), depending on the variable’s parametric or non-parametric distribution. So that, if our data followed a normal distribution, parametric tests were used; otherwise, non-parametric methods were used to compare them. P-values < 0.05 were considered as statistically significant.
Results
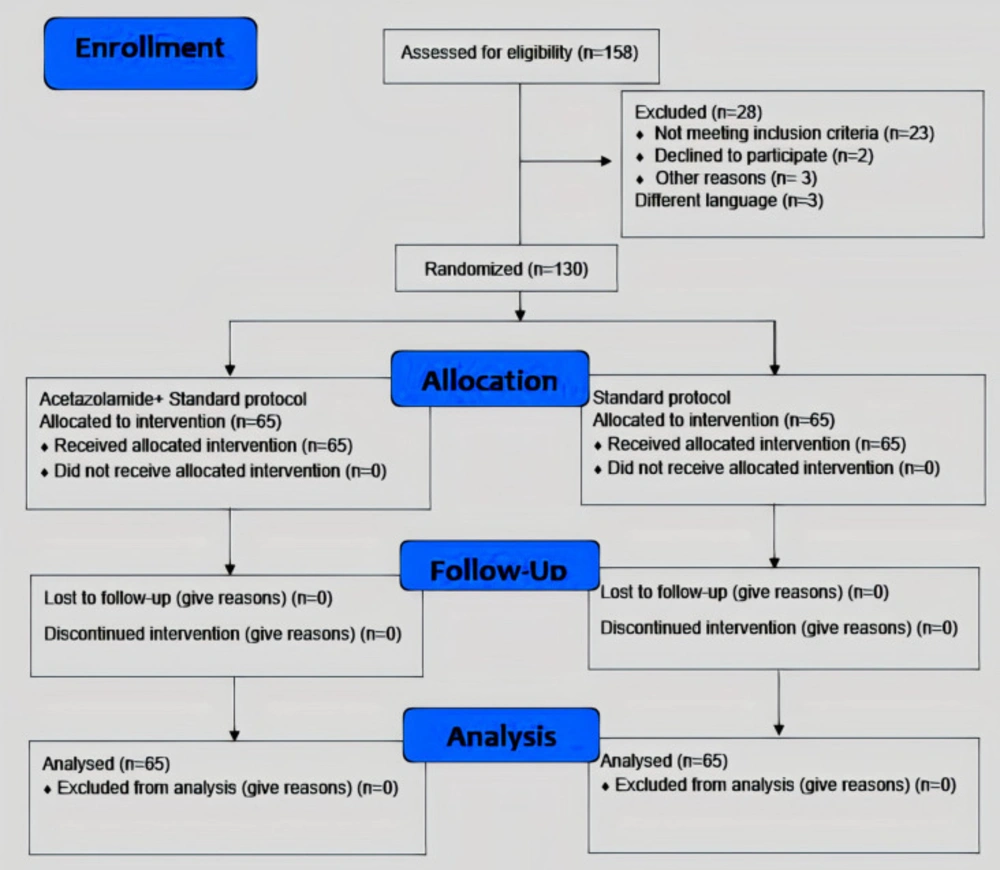
During the study period, 158 patients were assessed for eligibility; 28 of them were excluded from the study for not meeting inclusion criteria, declining to participate, or having a different language. Among 130 patients randomly assigned in both study groups (acetazolamide group, n = 65; control group, n = 65), all of them underwent all the study procedures (Figure 1).
No significant differences were found between the control and acetazolamide groups regarding baseline demographic and clinical characteristics, such as age, sex, body mass index (BMI), coexisting medical conditions, preoperative drugs, and LVEF (Table 2).
Table 3 shows biochemical data of the patients in the control and acetazolamide groups before and after CABG surgery. There were no significant differences in baseline SCr concentration, blood pH, and serum bicarbonate level between the two groups. Mean ± SD of SCr levels measured 24 h after CABG surgery was significantly lower in the patients who did not receive acetazolamide than those treated with it. Additionally, the mean absolute change of SCr concentration on the first day after CABG surgery was significantly higher in the acetazolamide group. Blood pH and serum bicarbonate levels were significantly lower in the acetazolamide group than the control group on the first day after CABG surgery.
As demonstrated in Table 4, AKI was diagnosed in 57 patients (43%). There was no significant difference in the incidence of AKI between the patients who received acetazolamide (44.61%) vs. the control group (43.07%) (P = 0.860). Patients who received acetazolamide had a significantly lower median length of hospital stay than those who did not receive it (8 days compared to 9 days, P = 0.006).
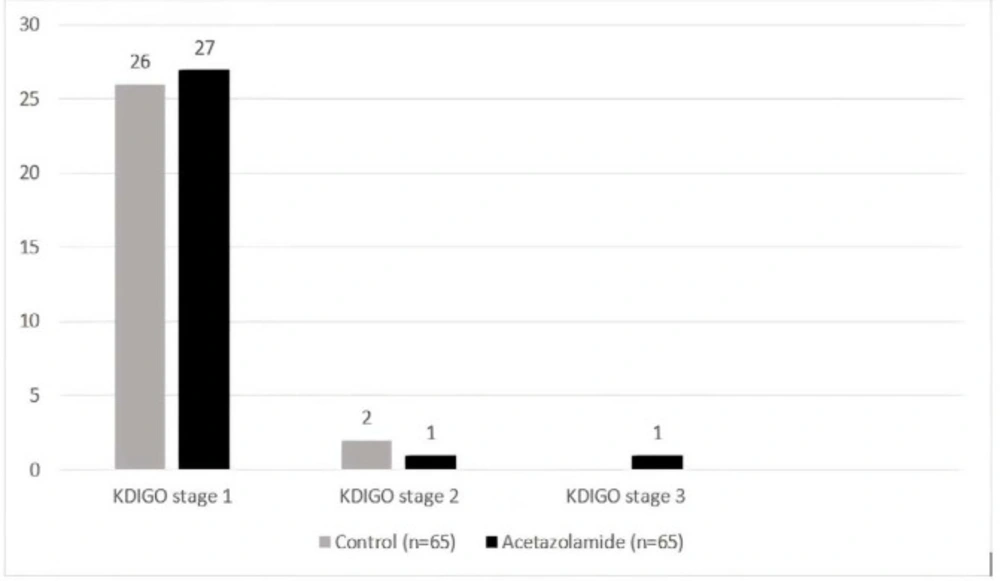
There was no significant difference in KDIGO AKI staging between the two groups (Figure 2). A total of 53 patients developed stage 1 of AKI. Among 29 patients in the acetazolamide group who developed AKI, SCr concentration was increased by 0.3 mg/dl or more within 48 h in 27 patients (93%). There was one patient in the acetazolamide group with AKI who progressed to stage 3.
Intra- and post-operative characteristics and complications of the patients in both groups are shown in Table 5. There was no difference in the need for inotropes, packed red blood cells, cross-clamping time, and cardiopulmonary bypass time between both groups.
There was no significant difference in post-operative LVEF, serum CRP level, adverse effects, and surgery complications between the two groups.
Discussion
In this randomized controlled trial, our results showed that post-operative AKI incidence was not significantly different between acetazolamide and control groups. Also, the need for mechanical ventilation, dialysis, admission to the ICU, or mortality rate did not differ significantly between the two groups. Length of hospital stay was significantly lower in the acetazolamide group.
The incidence of AKI, which occurred post-CABG surgery, is common and increases the risk of post-operative complications and mortality (20). Despite the advancements achieved in cardiac surgery and post-operative care, the incidence of AKI has increasingly become a great concern for all health professionals (21) as it is well acknowledged that to date, there are no pharmacological interventions proven to reduce the incidence of AKI after CABG surgery (22, 23). Therefore, the present study was conducted to evaluate the acetazolamide’s effects on ongoing kidney injury after CABG surgery.
Based on the results, there was no difference in the incidence of AKI between patients in the acetazolamide and the control group. The previously published studies have demonstrated that acetazolamide administration could be useful in preventing renal injury through different mechanisms, including reducing oxidative state, potential vasodilatory effect on renal circulation, and alkalinization of renal tubular fluid (16, 18 and 24-27). Shamash et al., showed that acetazolamide could play a renoprotective role by inhibiting renal tubular reabsorption of bicarbonate, inducing alkalinization, and decreasing precipitation of acidic drug in renal tubule (17). Additionally, Horita et al., (27) and Yu An et al., (18) reported that direct infusion of 1 g of acetazolamide into the patient’s main renal arteries and oral administration of 60 mg/kg/day of acetazolamide in mice could increase renal circulations by vasodilatory effect, respectively. This contrast in the results could be related to the difference in the route of administration (local infusion and intravenous (IV)), dosing, course, repeated exposure of acetazolamide, study population, and studying critically or stable ill individuals, which varied widely among these studies (27).
Surprisingly, our results showed that both SCr level and its change compared to baseline were significantly higher in the acetazolamide group than the control group on the first day after CABG surgery.
Several case reports have demonstrated an acute increase in SCr concentration following short courses of acetazolamide administration. All the cases have been presented with azotemia and back pain, as well as progressiveanuria. Those with radiographic evidence of intratubular obstruction revealed cellular debris, mucosal swelling, or blocking the ureters by sludge-like material.There was a full recovery of kidney function following cessation of acetazolamide intake, the removal of obstruction in all the individuals through IV or oral hydration, and avoidance of concurrent ischemic condition or the need for drugs, such as NSAIDs (28-31).
It is needless to say that occurrence of AKI after cardiac surgery is mostly secondary to a reduction in renal blood flow, leading to ischemic damages (32-34). The previous studies have demonstrated the role of carbonic anhydrase activity in acute recovery following renal ischemia-reperfusion injury (35, 36).
These findings may suggest two roles for carbonic anhydrase; the first one is the major role of carbonic anhydrase in decreasing ischemic injury by influencing renal oxygen homeostasis; the second one is related to natriuretic response to inhibit carbonic anhydrase and activation of tubuloglomerular feedback mechanism, as well as increasing proximal tubular pressure, leading to a decrease in renal blood flow, and glomerular filtration rate.
Therefore, it may not be reasonable to assert that acetazolamide administration before surgery is a nephroprotective method against ischemic injuries.
The previous studies have shown that acidic pH of tubular fluid influences ROS activity (16, 37, 38), resulting in an increase in the level of inflammatory markers and the exacerbated renal injury (39), as confirmed in the study by Assadi, who found that exposure to acetazolamide 2 h before and 12 h after the contrast agent administration could preferentially scavenge ROS by alkalinizing tubular fluid (16).
Since the effect of acetazolamide on inhibition of bicarbonate tubular reabsorption can be estimated simply by monitoring serum bicarbonate level (40). Coincidently, our results showed that serum bicarbonate level was significantly lower in the acetazolamide group than the control group just on the first day after CABG surgery, which could confirm alkalinization of renal tubular.
However, our study findings showed no significant difference in post-CABG CRP level between the two groups. As it seems to prevent activation of cytokine-induced inflammatory mediators, it may need to maintain alkalinization of tubular fluid status for longer periods.
There were some limitations in this study. Firstly, because of high cost and resource limitations regarding the use of parenteral acetazolamide in our institution, oral forms of the medication were used, which has a different bioavailability than its parenteral form. Second, since critical conditions could influence the absorption and bioavailability of acetazolamide, it is recommended to perform more clinical trials with varying acetazolamide doses to determine the precise dosage of this agent preventing AKI after CABG surgery. Also, it is recommended to conduct similar studies with a larger sample size to generalize the results to the other populations better.
| Stage of acute kidney injury | Serum Creatinine criteria | Urine output criteria |
|---|---|---|
| 1 | 1.5–1.9 times baseline | < 0.5 mL/kg/h for 6–12 h |
| ≥0.3 mg/dL (≥26.5 μmol/L) increase within 48 h | ||
| 2 | 2.0–2.9 times baseline | < 0.5 mL/kg/h for ≥12 h |
| 3 | ≥3.0 times baseline | < 0.3 mL/kg/h for ≥24 h |
| Increase in serum creatinine to ≥4.0 mg/dL (≥353.6 μmol/L) | Anuria for ≥12 h |
| Variables | Control (n = 65) | Acetazolamide (n = 65) | p-value |
|---|---|---|---|
| Age (year), mean ± SD | 61 ± 11 | 60 ± 10 | 0.706 |
| Sex (male), n (%) | 48(73.84) | 54(83.07) | 0.201 |
| body mass index (kg/m2), mean ± SD | 26 ± 3 | 26 ± 4 | 0.625 |
| Drug/food allergy, n (%) | 0 | 2(3.07) | 0.154 |
| Hypertension,n (%) | 38(58.46) | 35(53.84) | 0.596 |
| Diabetes, n (%) | 27(41.53) | 17(26.15) | 0.064 |
| Hyperlipidemia, n (%) | 20(30.76) | 21(32.30) | 0.850 |
| Ischemic heart disease, n (%) | 26(40) | 22(33.84) | 0.467 |
| Stroke, n (%) | 5(7.69) | 2(3.07) | 0.244 |
| Chronic lung disease, n (%) | 2(3.07) | 4(6.15) | 0.403 |
| Smoking, n (%) | 30(46.15) | 30(46.15) | 1 |
| Left ventricular ejection fraction %,median(25th,75th) | 50(40, 60) | 50(42, 55) | 0.962 |
| Beta-receptor blockers, n (%) | 25(38.46) | 21(32.30) | 0.463 |
| Calcium channel blockers, n (%) | 8(12.30) | 5(7.69) | 0.380 |
| ACE inhibitor, ARB, n (%) | 22(33.84) | 26(40) | 0.467 |
| Platelet inhibitors, n (%) | 35(53.84) | 38(58.46) | 0.596 |
| Statin, n (%) | 32(49.23) | 38(58.46) | 0.291 |
| Vasodilator, n (%) | 15(23.07) | 18(27.69) | 0.545 |
| Non-steroidal anti-inflammatory drugs (NSAIDs), n (%) | 8(12.30) | 16(24.61) | 0.07 |
| Variables | Control (n = 65) | Acetazolamide (n = 65) | P-value |
|---|---|---|---|
| Serum creatinine concentration, before | 1.2(1.2-1.3) | 1.18(1.17-1.30) | 0.341 |
| Serum creatinine concentration, Day1 | 1.33 ± 0.27 | 1.43 ± 0.25 | 0.036 |
| Serum creatinine concentration, Day2 | 1.33(1.32-1.50) | 1.36(1.32-1.52) | 0.998 |
| Serum creatinine concentration, Day3 | 1.22(1.22-1.38) | 1.15(1.13-1.43) | 0.074 |
| Serum creatinine concentration, Day4 | 1.18(1.15-1.29) | 1.10(1.09-1.37) | 0.172 |
| Serum creatinine concentration, Day5 | 1.18(1.11-1.23) | 1.10(1.05-1.30) | 0.181 |
| Serum creatinine concentration, Day6 | 1.16(1.09-1.21) | 1.07(1.04-1.28) | 0.181 |
| Serum creatinine concentration, Day7 | 1.12(1.12-1.23) | 1.06(1.05-1.28) | 0.109 |
| Serum creatinine changes base-day1 | -0.09(-0.13, 0.02) | -0.23(-0.26, -0.14) | 0.002 |
| Serum creatinine changes base-day2 | -0.09(-0.21, -0.05) | -0.11(-0.28, -0.09) | 0.615 |
| Serum creatinine changes base-day3 | 0.00(-0.10, 0.05) | 0.09(-0.18, 0.08) | 0.074 |
| Serum creatinine changes base-day4 | 0.08(-0.01, 0.12) | 0.12(-0.13, 0.13) | 0.247 |
| Serum creatinine changes base-day5 | 0.07(0.03, 0.17) | 0.13(-0.07, 0.17) | 0.303 |
| Serum creatinine changes base-day6 | 0.10(0.05, 0.18) | 0.13(-0.04, 0.19) | 0.498 |
| Serum creatinine changes base-day7 | 0.10(0.03, 0.16) | 0.15(-0.04, 0.17) | 0.208 |
| Blood Ph, Before | 7.34(7.30, 7.40) | 7.35(7.30, 7.40) | 0.772 |
| Blood Ph, 24 h after CABG surgery | 7.42(7.40, 7.47) | 7.42(7.38, 7.43) | 0.027 |
| Absolute change | -0.07 ± 0.08 | -0.05 ± 0.10 | 0.286 |
| Serum bicarbonate level, Before | 21.21 ± 2.75 | 20.94 ± 2.72 | 0.570 |
| Serum bicarbonate level, 24 h after CABG surgery | 25.27 ± 3.37 | 22.07 ± 3.43 | 0.00 |
| Absolute change | -4.20(-6.95, -2.5) | -1.30(-3.4, 1.2) | 0.00 |
| Outcome | Control (n = 65) | Acetazolamide (n = 65) | p-value |
|---|---|---|---|
| Acute kidney injury, n (%) | 28(43.07) | 29(44.61) | 0.860 |
| Need to post-operative RRT | 0 | 0 | - |
| Prolonged ventilation time (hour), median (25th,75th) | 12(8.5,18) | 11(8,21) | 0.573 |
| Mortality, n (%) | 7(10.76) | 4(6.15) | 0.344 |
| ICU length of stay (day), median (25th,75th) | 6(5,8) | 7(5,7) | 0.843 |
| Hospital length of stay (day), median (25th,75th) | 9(8,12) | 8(7,10) | 0.006 |
| Characteristic | Control (n = 65) | Acetazolamide (n = 65) | p-value |
|---|---|---|---|
| Anesthesia duration (hour), mean ± SD | 6.40 ± 1.19 | 6.07 ± 1.33 | 0.143 |
| Clamp time (minute), mean ± SD | 69 ± 28 | 72 ± 32 | 0.559 |
| Pump time (minute), mean ± SD | 120 ± 39 | 124 ± 46 | 0.649 |
| Albumin intake during surgery, n (%) | 51(78.4) | 50(76.9) | 0.833 |
| Albumin intake in ICU, n (%) | 43(66.1) | 45(69.2) | 0.708 |
| Packed cells during surgery, n (%) | 42(64.6) | 41(63.0) | 0.855 |
| Packed cells in ICU, n (%) | 29(44.6) | 44(67.6) | 0.08 |
| Need for inotropes in ICU, n (%) | 59(90.7) | 61(93.8) | 0.510 |
| LVEF after surgery %, median(25th,75th) | 50(40,55) | 50(45,55) | 0.981 |
| CRP level before surgery(mg/L),median (25th,75th) | 2(2,13) | 4(2,13) | 0.743 |
| CRP level 24 h after surgery (mg/L),median (25th,75th) | 13(6,24.5) | 16(8,26) | 0.131 |
| Change in CRP level (mg/L), mean ± SD | 8 ± 10 | 10 ± 12 | 0.312 |
| Mediastinitis, n (%) | 3(4.6) | 2(3.0) | 0.648 |
| Bleeding requiring surgical reintervention, n (%) | 4(6.1) | 2(3.0) | 0.403 |
| Thrombocytopenia, n (%) | 26(40) | 29(44.6) | 0.594 |
| Fatigue, n (%) | 5(7.6) | 3(4.6) | 0.465 |
| Skin allergy, n (%) | 3(4.6) | 6(9.2) | 0.30 |
Conclusion
Our findings revealed that administration of acetazolamide before CABG surgery did not reduce AKI incidence, duration of mechanical ventilation, length of ICU stays, and mortality rate. However, it was found that administration of acetazolamide can reduce the length of hospital stay in the patients undergoing CABG surgery. Although, further high-quality RCTs are needed to clarify the potential renoprotective effect of acetazolamide on AKI incidence after surgery.
Conflict of interest
The authors declare that they have no conflict of interests
Author contributions
AG: The first author, collected the cases, and (AG) has analyzed, drafted the manuscript. BS: analyzed the data using SPSS software program. AZ, BM, and KH participated in the design of the study and developed the research question. ZS: drafted the work and revised. DF: coordinated the study, participated in its conception and its design, and reviewed the manuscript. All authors contributed to and have approved the final manuscript.