Introduction
Although chemotherapy is one of the main treatment modalities for cancer, its use is restricted by different problems, such as nonspecificity and multidrug resistance (1). In recent years, nanotechnology-based drug delivery systems have been widely used to improve the treatment outcome of chemotherapy (2, 3). Nanosized delivery systems cause a major change in the pharmaceutical field by improving the therapeutic efficacy and bioavailability of most drugs (4). In this regard, various drug delivery systems, such as liposomal NPs, polymeric micelles, dendrimers, carbon nanotubes, aptamers, quantum dots, and polymer compounds have been developed (5). Polymeric and lipidic NPs are two US Food and Drug Administration (FDA) approved compounds for clinical use and have been successfully used for the encapsulation and release of various drugs in the past two decades (6, 7). The use of amphiphilic polymers for the construction of polymeric NPs leads to the formation of NPs with a hydrophobic core and a hydrophilic shell. The core-shell-based NPs can encapsulate and deliver the poorly water-soluble drugs and increase their blood half-life. Also, they can release the cargoes at a steady rate in the optimal range of drug concentration (8, 9).
Liposomes are spherical lipid vesicles with a bilayer structure of synthetic or natural amphiphilic lipid molecules. They have been widely used in nano-drug delivery systems due to their appropriate features, such as proper safety properties and long blood circulation time, which can be achieved by surface modification using hydrophilic polymers such as polyethylene glycol (PEG) (10). PEG causes an increase in the molecular weight of conjugates and improves the aqueous solubility as well (11).
Overall, polymeric NPs take advantage of high drug loading efficiency, controlled drug release profile, appropriate stability in the blood compartment, and high cellular uptake. However, the biocompatibility and circulation half-life of polymeric NPs are two main concerns that must be considered. In contrast, liposomes possess higher biocompatibility and prolonged circulation profile in the blood compartments. Moreover, their surface can be easily modified. Thus, it would be interesting to combine the advantages of liposomes and polymeric NPs to develop an advanced hybrid therapeutic system. These systems (HLPNPs) can be produced by coating the polymeric NPs with lipid layers (12). HLPNPs consist of three distinct compartments, including 1) inner hydrophobic core as a drug supplier, 2) interfacial lipid layer as an exceptional biocompatible layer, and 3) outer hydrophilic polymer shell comprising of PEG to increase the blood circulation time. These NPs have high structural integrity, biocompatibility, and appropriate pharmacokinetic profile which result in an improvement in the anticancer efficacy of chemotherapeutics (13).
In this study, melphalan-loaded HLPNPs were synthesized and the drug delivery system was optimized using a central composite design (CCD) which is one of the elements of response surface methodology (RSM) (14). Melphalan is a hydrophobic anticancer drug used for the treatment of ovarian cancer (15, 16). The system (HLPNPs) utilized poly lactic-co-glycolic acid (PLGA) as the polymer to encapsulate melphalan. PLGA was used due to its biodegradability and a high potential for loading hydrophobic drugs. Phosphatidylcholine was also used as the lipid constituent for coating the polymer core and as a biological membrane to improve the penetration of the NPs (17). In addition, polyvinyl alcohol (PVA) was used as the surfactant. The effects of various variables (PLGA, lipid, and PVA) were evaluated on the size and drug entrapment efficiency (EE %) and the optimized formulation was characterized in terms of drug release and cytotoxicity effects. This study aimed to investigate the cytotoxic efficacy of the NPs loaded with melphalan. Also, it was assumed that using HLPNPs enhances the therapeutic effects of melphalan.
Experimental
Materials
Melphalan was purchased from CELON LAB Co. (India). Soya lecithin, methyl thiazole tetrazolium (MTT), and polyvinyl alcohol (PVA) were prepared from Sigma-Aldrich Co (Germany). Roswell Park Memorial Institute-1640 (RPMI-1640), and Dulbecco’s Modified Eagle’s Medium (DMEM) were purchased from Invitrogen (Germany). Human ovarian cancer A2780CP and SKOV3 cells were obtained from the Pasteur Institute of Iran. Moreover, organic solvents such as chloroform, methanol, and isopropanol were purchased from Merck Co (Germany). Furthermore, (PLGA) at 50:50 molar ratio was purchased from Iran Polymer and Petrochemical Institute. Deionized water was used to prepare the NPs. All of the materials used were of analytical grade.
Experimental design
The amount of lipid, polymer, and PVA used for the construction of HLPNPs, was optimized using an experimental design by evaluating their effects on the physical and chemical properties of the HLPNPs. For this purpose, 20 experiments were designed using RSM and CCD and Design Expert 10.0.7 Trail software. The effects of three factors [Polymer (A), Lipid (B) and PVA (C)] as independent variables at three different levels (1, 0, -1), two axial points (-α, +α), and six replicates at the central point were studied to estimate the trial error and calculate repeatability. Physical and chemical properties, such as particle size (Y1) and drug EE% (Y2) were selected as the dependent variables. Table 1 presents the design parameters. The optimum condition was considered when the particle size (Y1) was minimum while the drug EE% (Y2) was the maximum (18).
Fabrication and preparation of the HLPNPs drug
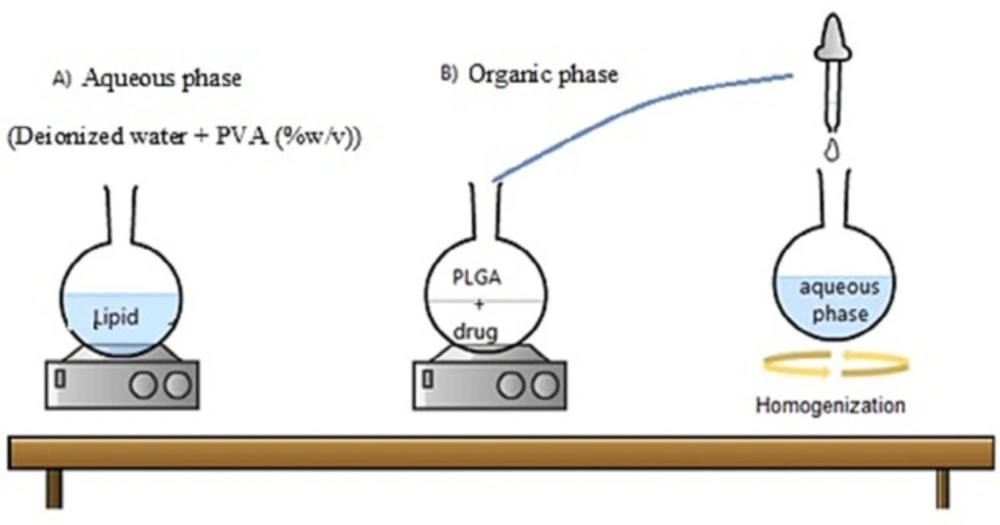
NPs were prepared using the nanoprecipitation method (13). Briefly, different amounts of lipid (2.3-5.7 mg/mL) in an aqueous solution containing different amounts of PVA (0.3-3.7% V/W) were used to prepare the aqueous phase. Also as the organic solvent, different amounts of polymer (3.3-6.7 mg/mL) were dissolved at the constant content of the drug (1 mg/mL) in chloroform. Then, the organic phase was added dropwise (to avoid aggregation) to the aqueous phase under high stirring and ice bath conditions (using a homogenizer at a speed of 12000 rpm). The obtained solution was stirred at ambient temperature for 2 h to make the binding and remove the organic solvent. To ensure that any organic solvent was removed, the obtained nanosuspension was centrifuged three times by Amicon filter 12 kDa. In the next stage, the obtained precipitation was suspended again in deionized water to examine their size and shape. Afterward, the resulting solution was placed on ice and sonicated (5 min, 60 HZ) to obtain more homogeneous NPs.
HLPNPs Specifications
Particle size, polydispersity index (PDI), and zeta potential were determined using the Zetasizer device (Nano ZS3600, Malvern Instrument Ltd, UK) based on the method described previously (19). The samples were placed in the analysis cell. The experiment was conducted at ambient temperature with a 90° detection angle. Each experiment was repeated three times, and their responses (nanoparticle size) were measured. Finally, the mean value of each measurement and standard deviation (SD) were calculated.
Nanoparticles morphology
Transmission electron microscopy (TEM) (Hitachi, Japan, H9500 (JAPE)) was used to detect the structure of the prepared NPs. For this purpose, 1 mg/mL of the nanoformulation was prepared in PBS, from which a drop was injected into the device, and the morphology of the nanoliposome on the sample was investigated by producing high-energy electron beams.
Determination of the drug entrapment efficiency
To evaluate the amount of the encapsulated drug, 2 mL of each formulation (HLPNPs and its blank sample) was centrifuged (Ultra Centrifuged –UCEN, Iran) at 20,000 rpm and 4 °C for 30 min. The supernatant was obtained and the optical absorbance of the supernatant of each formulation was read by spectrophotometer (UV-160 IPC, Shimadzu, Japan) at the wavelength of 260 nm (20). The following equations were used to calculate the encapsulation and loading efficiencies of melphalan. (21):
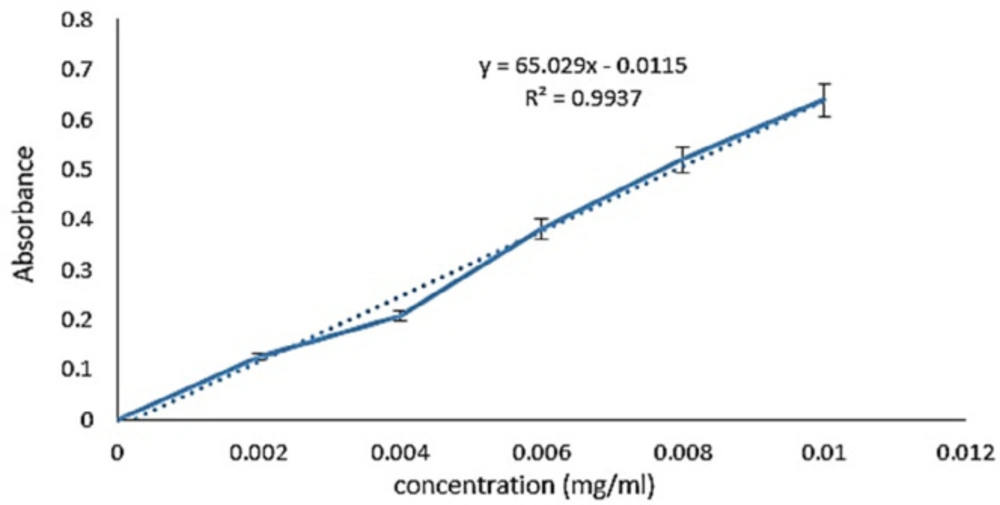
To obtain the standard curve, different concentrations of melphalan were prepared by serial dilution method. The light absorption of the samples was read at 260 nm, and the results were plotted as a standard curve using excel software (Figure 1). The results were obtained from at least three independent experiments.
Analytical method validation parameters
In this study, validation parameters of the melphalan examination containing range (2-10 µg/mL), linearity, and limit of quantification (LOQ) were investigated. Equation 3 was applied to calculate LOQ and its value computed for melphalan using the information of calibration curve (22):
Where σ and S are the standard deviation of the response and the slope of the calibration curve respectively.
Evaluation of melphalan release from the hybrid nanoparticles
The dynamic diffusion method of the dialysis membrane was used to measure the drug release in vitro (23). Two mL of optimal formulation, free drug, and its blank sample were separately poured into three dialysis bags (Mw: cutoff 12 KDa). Then, the dialysis bags were separately immersed into vessels containing 25 mL of phosphate buffer saline (PBS, pH 7.4), and stirred for 48 h (200 rpm and 37 °C). At different time intervals, 2 mL of PBS was replaced with 2 mL of the fresh buffer. The absorbance of the samples was measured at 260 nm, and the drug release was determined.
Evaluation of the cytotoxic effect of the formulations
Cytotoxicity of the HLPNPs of melphalan and the free drug was evaluated by MTT assay on ovarian cancer cell lines (A2780CP and SKOV3). A2780CP and SKOV3 cells were initially cultured in RPMI-1640 and DMEM, respectively. Then, 100 µL of the suspension containing 104 cells was transferred into 96-wells and incubated at 37 ℃ in a 5% CO2 incubator. After 24 h, the culture media was removed and the media containing different concentrations (4.25, 8.5, 17, 34, and 68 µg/mL) of melphalan-loaded HLPNPs, its control, and the standard drug were added to the wells and incubated for 48 and 72 h. Afterward, the culture medium was discarded, and 100 μL of MTT solution (0.5 mg/mL) was added and incubated for 3 h (37 °C, 5% CO2). Next, the MTT solution was removed, and 100 μL isopropanol was added to dissolve the formazan crystals. The absorbance of the samples was then read at 570 nm by an ELISA reader (Bio Tek. Instrument, USA). The amount of the half-maximal inhibitory concentration (IC50) was calculated by using the statistical Graph-pad prism program.
Moreover, Equation 4 (24) was used to calculate cell viability. The results were obtained from three independent experiments.
Statistical analysis
All results are expressed as mean ± standard deviation (SD, n = 3). The data were analyzed and evaluated by ANOVA using Design Expert 7 software, and p-value < 0.05 was considered to be statistically significant, with a 95% confidence interval.
Result and Discussion
Mechanism of the single-step nanoprecipitation method for the production of the hybrid nanoparticles
The nanoprecipitation method is one of the fast and repeatable ones for preparing the HLPNPs (Figure 2) (25). This method was mainly based on the polymer precipitation from the lipophilic solution which was a combination of polar solvent and water. In particular, the used polymer (PLGA) was precipitated as a hydrophobic core to encapsulate the less water-soluble drug. Notably, adding a lipid layer between the PLGA polymer core and PEG shell results in i) restriction of the release of small drug molecules from the polymer core which improves the encapsulation and loading efficiencies, and ii) reduction of the water penetration into the polymer core, and as a result, reduction in the hydrolysis rate of the PLGA polymer which causes a slow drug release from the NPS.
Validation parameters
Based on the linear equation taken from the melphalan calibration curve the values of σ and S were (0.0346 and 65.02 respectively). Consequently, according to Equation 3, the amount of LOQ was obtained at 5.32 µg/mL.
Analysis and optimization of the central composite design
Statistical analysis based on RSM was used to predict the most appropriate model to describe the response surfaces (nanoparticle size and EE%). Each experiment was repeated three times, and the response surface was determined in each experiment. Table 2 presents the outputs. The results of the experimental design indicated that the designed system was affected by the amount of lipid, polymer, and PVA, resulting in various drug EE% and NPs sizes. As shown in Table 3 and Table 4, the best state for each quadratic model response compared to the linear model was the quadratic two-factor model, which had the highest correlation coefficient (R2). Thus the quadratic model was selected to describe binary interactions of independent variables on each response.
Effect on the size of the nanoparticles
The NPs size is imperative to determine the efficiency of loaded therapeutics where NPs with a smaller size have a higher chance to internalize into cells, resulting in the higher intracellular concentration of the loaded therapeutics (26). NPs with a size smaller than 300 nm are effectively up taken by target cells and exert their pharmaceutical activity (27).
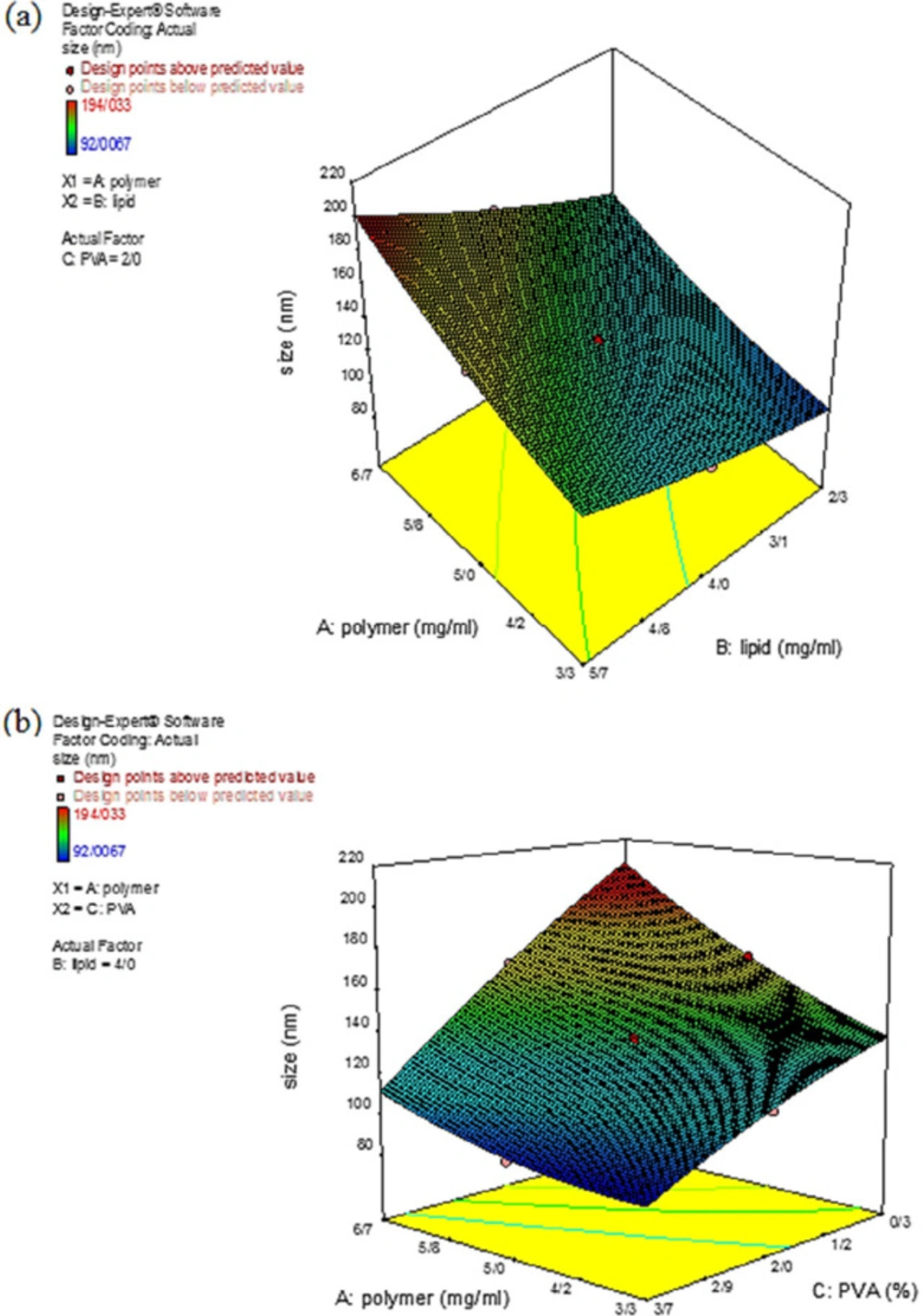
In the present study, the NPs size for the 20 synthesized formulations was in the range of 92.01 – 194.03 nm. According to Table 2, the smallest and largest NPs were produced in experiments 20 and 10, respectively. The proposed quadratic model to describe the effect of the independent variables on the particle size is presented in Equation 5:
Statistical results demonstrated that by increasing the amount of polymer (A) and lipid (B), the particle size with positive coefficients also increased, as shown in Equation 5.
Analysis of variance in the statistical calculations does not express the results with 100% certainty, thus the results are expressed with a percentage of probability. Therefore, by increasing the F-value and decreasing the P-value, the importance of the NPs size would be significant and could be considered as a parameter affecting the process. Accordingly, concerning the results from data variance in Table 5, all three independent variables were found to affect the NPs size. Figure.3 depicts the three-dimensional (3D) curve of the particle size response surface for a better understanding of the binary interaction of the independent variables on the response surface. According to Figure.3a, at the high polymer concentrations, the particle size was risen by increasing the lipid concentration. This increase in the particle size might be due to increasing the viscosity of the solution, which in turn caused an increase in the liquid phase resistance of the particle dispersion. Consequently, the particle size can be increased by increasing the interconnection of particles among each other. Therefore, by increasing the number of particles, the interconnection rate between particles was increased, resulting in the production of larger NPs (28, 29). Also, by increasing the viscosity, the evaporation rate of the organic solvent was decreased, and particles with larger sizes were produced (30, 31). Figure 3b depicts that by increasing the initial concentration of the surfactant, the particle size decreased. This decrease in the particle size could be explained by the fact that at high concentrations of surfactant, the surfactant molecules tend to be accumulated and were sufficient to coat the NPs. Hence, the surfactant activity increased and exert a significant effect on the NPs size (32, 33).
Effect on the drug entrapment efficiency
Encapsulation efficiency is a critical factor that affects the efficacy of the drug delivery carrier (34). For example, low drug entrapment efficiency in polymeric carriers causes poor treatment outcomes and drug release properties and, as a result, insufficient efficacy of drug delivery systems (19).
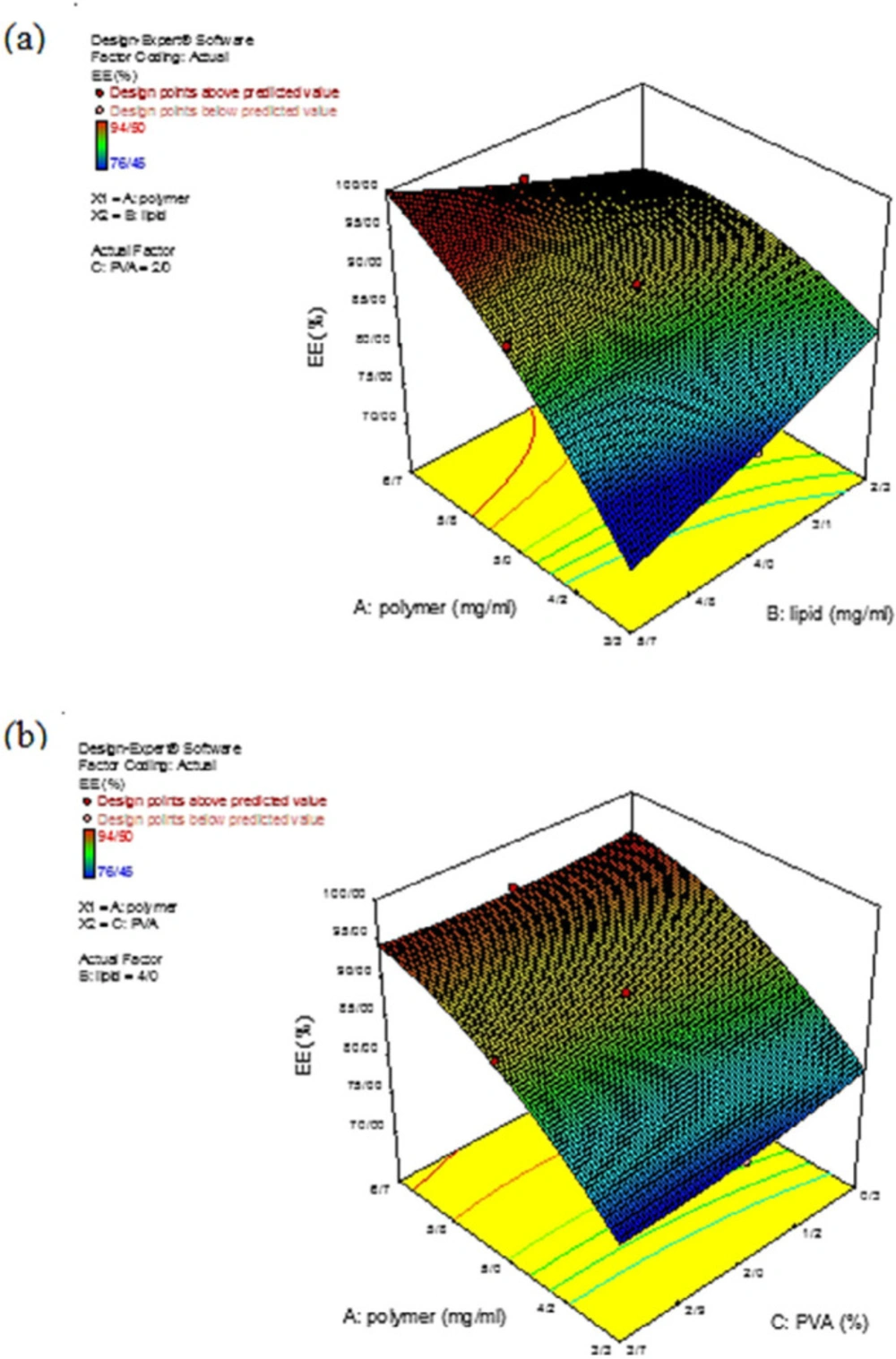
In the present study, EE% was calculated for all the prepared formulations to evaluate the effects of the independent variables (polymer, lipid, and PVA) on the drug EE% at different concentrations where the drug concentration was constant. Variation in the EE% at different concentrations was described by Equation 6:
According to Table 2, Experiments number 10 and 14 exhibited the highest and lowest EE%, respectively. Regarding the importance of the F-value parameter and statistical results reported in Table 6, the two independent variables of polymer concentration (A) and amount of surfactant (C) had significant effects on the EE% (p-value < 0.05). Figure 4 demonstrates the impacts of the independent variables on the drug EE%. As can be seen in Figure 4a, increasing the polymer concentration, the EE% also increased. This could be a result of the fact that by increasing the polymer concentration, the encapsulation spaces for the drug also increased; hence, a relatively compressed matrix was created. Also, the hydrophobicity of melphalan helped to achieve a high EE%. According to the previous research, polymeric core and the drug hydrophobicity, as the two significant factors, promoted the EE%. Furthermore, changes in the lipid content had no significant effect on EE% and only affected the lipid thickness which resulted in a partial drug release from the nanoparticle (6). As Figure.4b demonstrated that by decreasing the polymer concentration and increasing the surfactant content, the EE% decreased. These results from the fact that with increasing the surfactant concentration, the solubility of the drug from the organic phase to the aqueous phase increased, which caused a reduction in the viscosity, and consequently, a reduction in permeation during the process. This issue, in turn, led to a decrease in EE% (35).
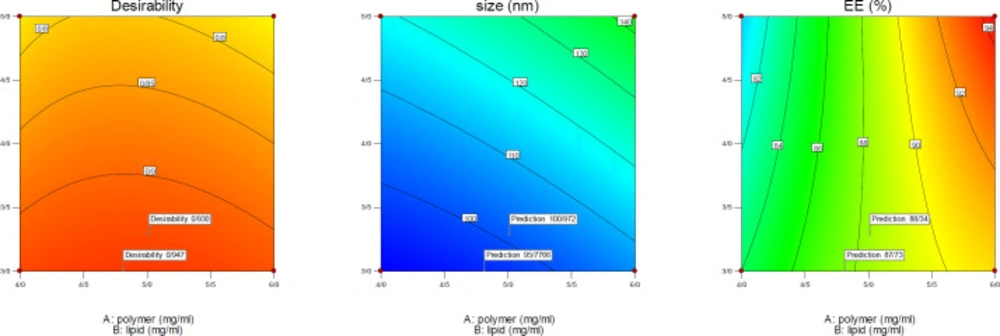
Optimization
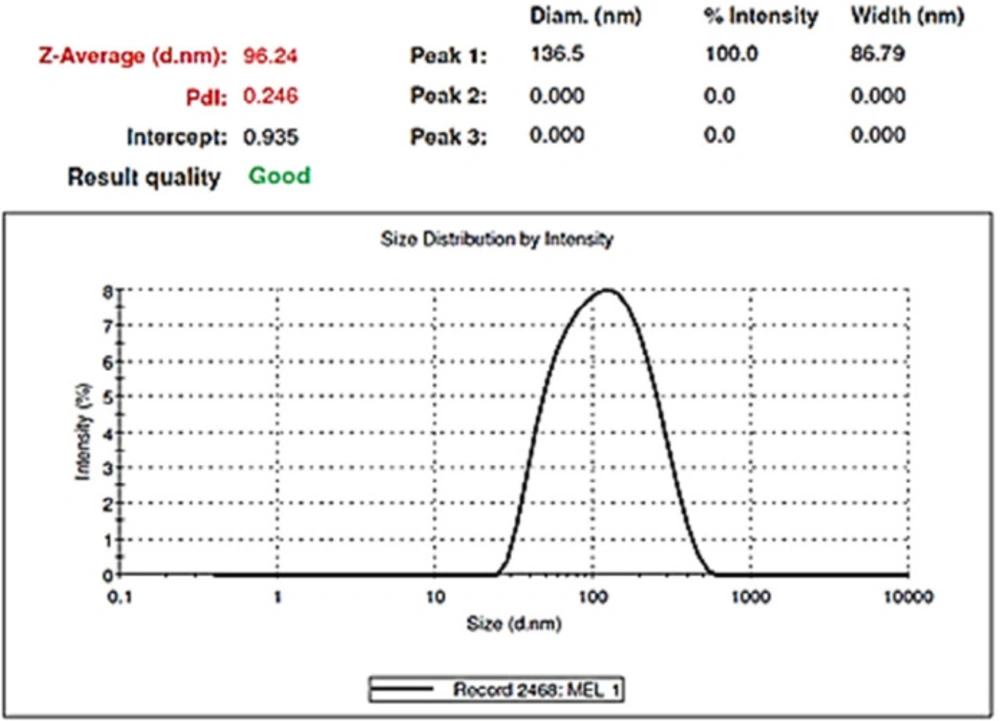
After evaluating the response surfaces by analyzing variance, numerical optimization was performed by applying optimal limitations and specifications for independent variables. Then optimal response surfaces were obtained with the values predicted by the software (Figure 5). Then, the formulation of the HLPNPs with the predicted values was prepared, and the NPs size, PDI (Figure 6), and EE% were measured and calculated. Table 7 reports values predicted by the software and actual values of the response surfaces. According to the data presented in Table 7, there was no significant difference between the actual values of the response surfaces and the predicted values. Therefore, the efficiency of encapsulation and loading of melphalan was obtained to be 84.43 ± 3.67% and 2.98 ± 1.3%, respectively.
Moreover, the desirability of the optimized values was 0.947. In general, it could be concluded that those NPs used as a carrier for anticancer drugs, can easily reach the cell membrane and increase the drug concentration at the cell surface compared to the standard drug. consequently, such a condition increased the therapeutic effect of the anticancer drug. Hence, a little encapsulation efficiency would be of high importance.
Morphology and zeta potential of the optimal formulation
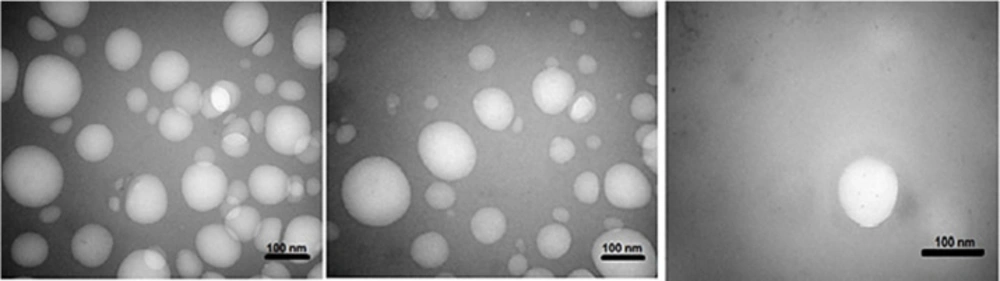
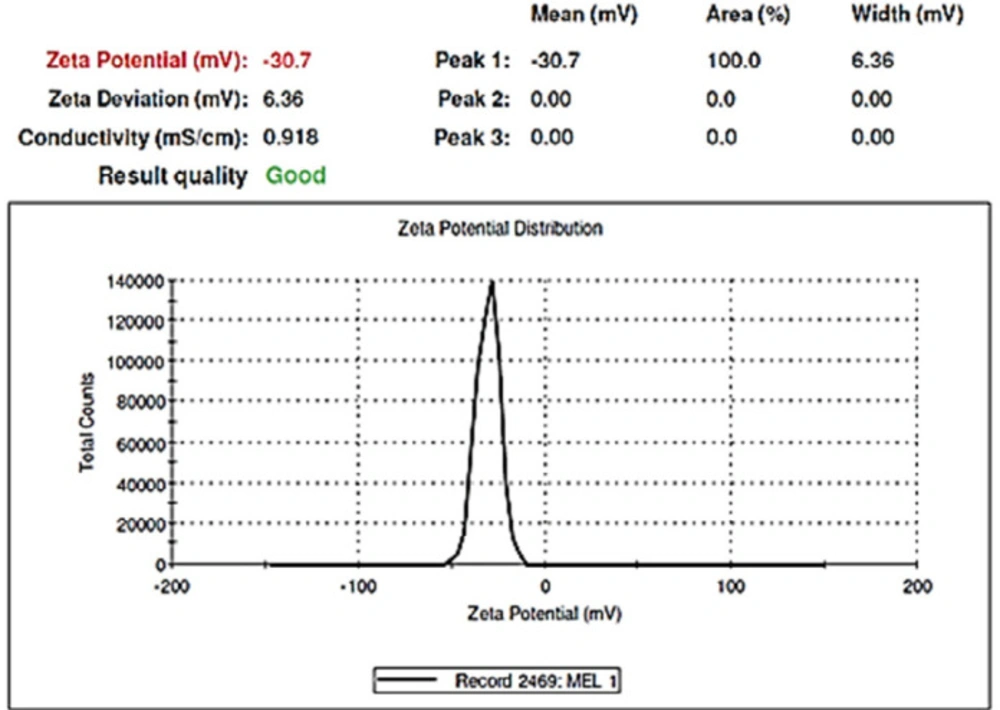
TEM was used to identify the morphology of the optimized hybrid NPs. Figure 7 shows images obtained from TEM, indicating a small ring of lipid covering around the polymer core. Moreover, it could be seen that the prepared NPs had a smooth surface, uniform, and integrated pattern with the spherical structure, suggesting a slow release of the drug. There was a difference, but not significant, between the particle size obtained from TEM (94.41 nm) and those obtained by zeta sizer (96.24 nm) (Figure 6). These results were somewhat consistent with the results of similar research, reported previously (36, 37). Zeta potential determines the stability of the colloidal nanosuspensions. Figure 8 depicts the zeta potential of the optimized value attributed to the nonionic nature of PVA compared to the anionic nature of phosphatidylcholine. Additionally, negative zeta potential created a large repulsive force between NPs, prevented their aggregation, and resulted in their stability (38).
Drug release study (in-vitro)
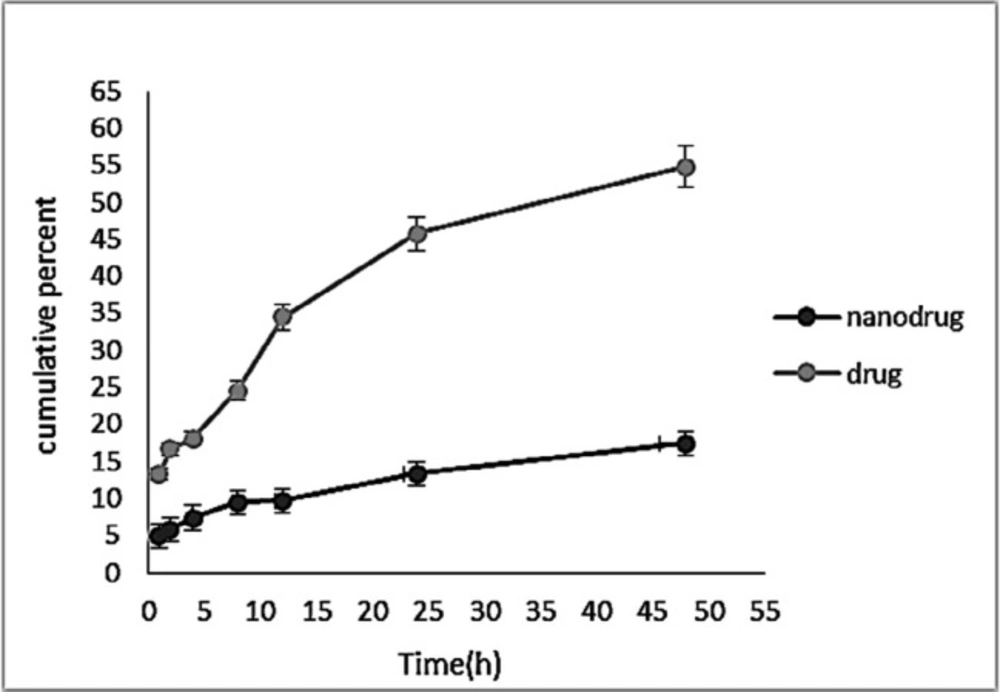
Controlled-release drug delivery systems have remarkable advantages in comparison with conventional dosage forms. These systems 1) cause a significant decrease in the dosing frequency and provide more convenience for patients, 2) cause a minimum in the fluctuation of drug concentration in-vivo and preserve the concentration of drugs within the proper range, 3) can deliver drugs site-specifically, and 4) can reduce the drug side effects (19). Also, drug release from nanocarriers is a critical factor affecting the therapeutic outcome (39). Figure 9 demonstrates a pattern of sustained melphalan release (for both the standard and encapsulated forms) at any time point. According to the Figure, the rate of drug release from the NPs was much lower than that of the free drug release, indicating that the NPs were able to encapsulate the drug, and release it in a controlled manner in that only 17.39% of the encapsulated drug was released after 48 h. The drug release from the NPs was initiated with a burst release, in which 29% of the total release occurred in the first hour of the study. This could result from the release of the adsorbed drug to the NPs surface. Releasing continued at a reduced rate until the end of the study. Lack of the rapid release of the drug from the NPs suggested the proper interaction of melphalan with HLPNPs. Overall, the pattern of drug release from the NPs indicated the potency of the particles as a controlled drug delivery system.
In-vitro cell viability and IC50 of nanoformulation
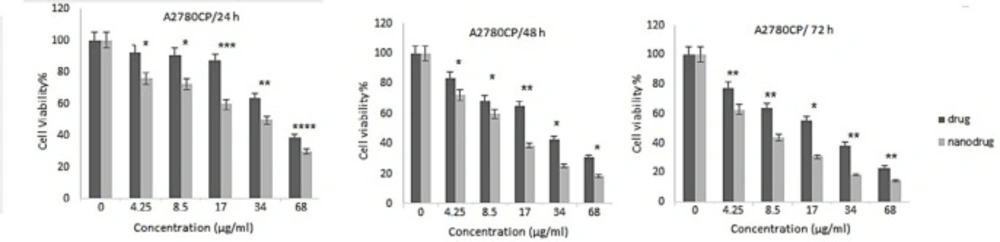
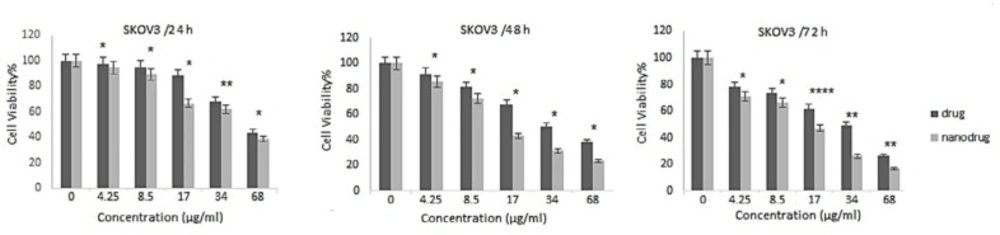
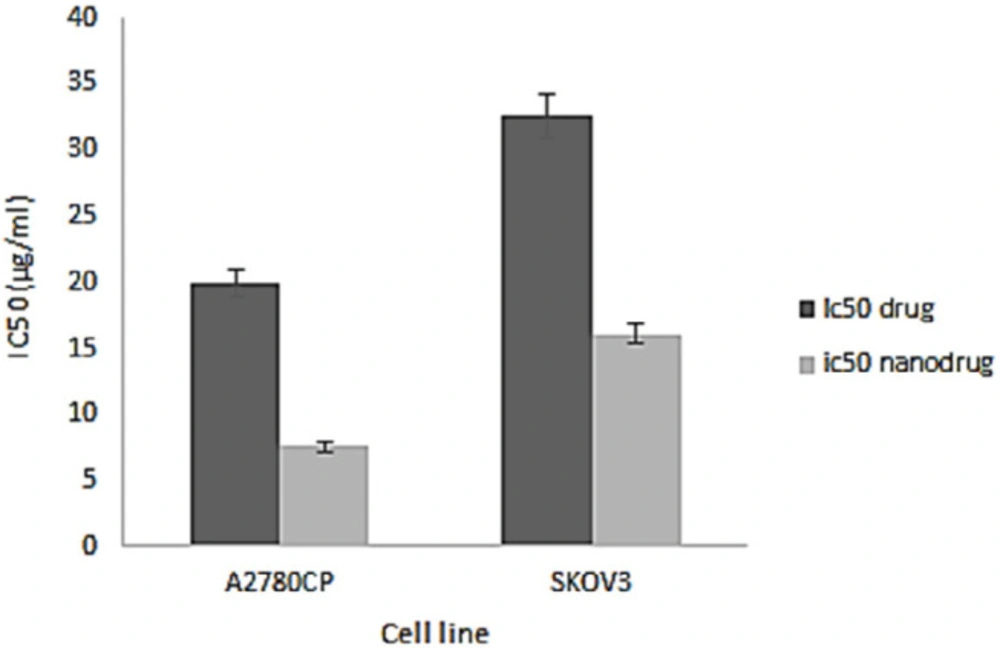
NPs can increase the therapeutic effects of anticancer drugs because of their capability to enhance the drug concentration in tumor cells. NPs perform this by increasing drug circulation time. Moreover, NPs can deliver drugs site-specifically in-vivo, resulting in the restriction of the drug side effects (26). In the present study, the cytotoxicity effects of melphalan and melphalan-loaded HLPNPs against human ovarian cancer A2780CP and SKOV3 cells were evaluated. As melphalan is used for the treatment of ovarian cancer; therefore, A2780CP and SKOV3 cells were used as in-vitro models of the disease. The results demonstrated that the cytotoxicity of both formulations (melphalan and melphalan-loaded NPs) was increased in a dose-dependent manner (Figures 10 and 11). Also, the cytotoxicity was found to be cell type-dependent, as both formulations caused higher cytotoxicity in A2780CP cells compared to SKOV3 cells. However, melphalan-loaded HLPNPs were more potent compared to the standard drug (at the same drug concentration) to inhibit the growth of cancer cells, indicating the potency of the HLPNPs to increase the cytotoxicity effects of melphalan. Increasing the cytotoxicity effects of the melphalan-loaded NPs compared to melphalan, resulted from the controlled drug release from the HLPNPs. Moreover, the nanoformulation at high concentrations inhibited the toxic effects of melphalan. This feature increases the maximum tolerable dose, and thus higher concentrations of the drug can be used, which in turn decreases the risk of tumor drug resistance. The cytotoxicity effects were also evaluated by calculating the half-maximal inhibitory concentration (IC50): the drug concentration required to kill 50% of the cells incubated over the determined period). Figure 12 shows the IC50 values calculated for both cell lines over the intended time in comparison to the free drug. As seen in the Figure, the IC50 values of the HLPNPs were lower than that of the free drug over time because the free drug quickly passed through the cell membrane while the encapsulated form of the drug chose a specific pathway and released the drug in a controlled way.
| Independent variables Levels | |||||
|---|---|---|---|---|---|
| -1.68 | -1 | 0 | 1 | 1.68 | |
| A: Polymer (mg.mL) | 3.3 | 4 | 5 | 6 | 6.7 |
| B: Lipid (mg.mL) | 2.3 | 3 | 4 | 5 | 5.7 |
| C: PVA (%) | 0.3 | 1 | 2 | 3 | 3.7 |
| Dependent variables | Constrains | ||||
| Y1: Particle size (nm) | Minimize | ||||
| Y2: Entrapment efficiency (%) | Maximize | ||||
| Std | Run | Polymer(mg.mL) | lipid(mg.mL) | PVA (%) | Size(nm) | EE (%) |
| 10 | 5 | 1.682 | 0 | 0 | 162.85 ± 5.5 | 94.5 ± 2.2 |
| 4 | 10 | 1 | 1 | -1 | 194.03 ± 2.9 | 94.45 ± 2.5 |
| 8 | 12 | 1 | 1 | 1 | 144.07 ± 3.5 | 93.85 ± 1.6 |
| 2 | 13 | 1 | -1 | -1 | 156.1 ± 5.1 | 91.5 ± 1.1 |
| 6 | 17 | 1 | -1 | 1 | 107 ± 2.4 | 90.55 ± 1.8 |
| 20 | 1 | 0 | 0 | 0 | 134.29 ± 2.6 | 88.45 ± 2.4 |
| 11 | 2 | 0 | -1.68 | 0 | 108.69 ± 1.9 | 88.45 ± 1.3 |
| 17 | 4 | 0 | 0 | 0 | 136.13 ± 1.1 | 88.65 ± 1.7 |
| 13 | 7 | 0 | 0 | -1.68 | 165.74 ± 5.1 | 90.01 ± 2.1 |
| 16 | 8 | 0 | 0 | 0 | 134.29 ± 2.5 | 87.89 ± 1.4 |
| 18 | 11 | 0 | 0 | 0 | 133.77 ± 3.3 | 88.5 ± 1.12 |
| 15 | 16 | 0 | 0 | 0 | 134.26 ± 2.8 | 88.4 ± 2.5 |
| 12 | 18 | 0 | 1.68 | 0 | 164.46 ± 3.1 | 89.5 ± 1.6 |
| 19 | 19 | 0 | 0 | 0 | 132.32 ± 3 | 89 ± 3.1 |
| 14 | 20 | 0 | 0 | 1.68 | 92.01 ± 2.4 | 88.58 ± 1.2 |
| 3 | 3 | -1 | 1 | -1 | 154.75 ± 4.4 | 81.48 ± 2.3 |
| 7 | 6 | -1 | 1 | -1 | 120.19 ± 3.3 | 80 ± 2.5 |
| 1 | 9 | -1 | -1 | -1 | 124.88 ± 4.1 | 86.5 ± 1.2 |
| 5 | 15 | -1 | -1 | 1 | 94.24 ± 2.6 | 84.45 ± 1.2 |
| 9 | 14 | -1.68 | 0 | 0 | 114.36 ± 1.8 | 76.45 ± 2.3 |
| Y1( size(nm)) | |||||||
|---|---|---|---|---|---|---|---|
| Source | Std.Dev. | R-Squared | Adjusted R-Squared | Predicted R-Squared | p-value | ||
| Linear | 4.65 | 0.9728 | 0.9677 | 0.9506 | 0.0018 | ||
| 2FI | 3.45 | 0.9879 | 0.9823 | 0.9679 | 0.0067 | ||
| Quadratic | 1.84 | 0.9973 | 0.9949 | 0.9832 | 0.0953 | Suggested | |
| Cubic | 1.95 | 0.9982 | 0.9943 | 0.7333 | 0.0239 | Aliased | |
| Y2 (EE%) | ||||||
|---|---|---|---|---|---|---|
| Source | Std.Dev. | R-Squared | Adjusted R-Squared | Predicted R-Squared | p-value | |
| Linear | 1.85 | 0.8631 | 0.8375 | 0.7498 | 100.16 | |
| 2FI | 1.34 | 0.9418 | 0.9149 | 0.8722 | 51.17 | |
| Quadratic | 0.62 | 0.9905 | 0.982 | 0.9379 | 24.87 | Suggested |
| Cubic | 0.33 | 0.9984 | 0.9948 | 0.9957 | 1.72 | Aliased |
| Source | Sum of | df | Mean | F | p-value | |
|---|---|---|---|---|---|---|
| Model | 12698.91 | 9 | 1410.99 | 414.75 | < 0.0001 | significant |
| A-polymer | 2607.24 | 1 | 2607.24 | 766.38 | < 0.0001 | |
| B-lipid | 3694.58 | 1 | 3694.58 | 1086.00 | < 0.0001 | |
| C-PVA | 6084.58 | 1 | 6084.58 | 1788.53 | < 0.0001 | |
| AB | 46.04 | 1 | 46.04 | 13.53 | 0.0043 | |
| AC | 143.31 | 1 | 143.31 | 42.12 | < 0.0001 | |
| BC | 2.86 | 1 | 2.86 | 0.84 | 0.3811 | |
| A2 | 59.30 | 1 | 59.30 | 17.43 | 0.0019 | |
| B2 | 24.79 | 1 | 24.79 | 7.29 | 0.0223 | |
| C2 | 28.71 | 1 | 28.71 | 8.44 | 0.0157 | |
| Residual | 34.02 | 10 | 3.40 | |||
| Lack of Fit | 26.55 | 5 | 5.31 | 3.55 | 0.0953 | not significant |
| Pure Error | 7.47 | 5 | 1.49 | |||
| Cor Total | 12732.93 | 19 |
| Source | Sum of | df | Mean | F | p-value | |
|---|---|---|---|---|---|---|
| Model | 396.60 | 9 | 44.07 | 115.88 | < 0.0001 | significant |
| A-polymer | 341.34 | 1 | 341.34 | 897.60 | < 0.0001 | |
| B-lipid | 0.15 | 1 | 0.15 | 0.41 | 0.5378 | |
| C-PVA | 4.10 | 1 | 4.10 | 10.79 | 0.0082 | |
| AB | 30.89 | 1 | 30.89 | 81.23 | < 0.0001 | |
| AC | 0.49 | 1 | 0.49 | 1.29 | 0.2828 | |
| BC | 0.11 | 1 | 0.11 | 0.28 | 0.6094 | |
| A2 | 16.50 | 1 | 16.50 | 43.39 | < 0.0001 | |
| B2 | 0.40 | 1 | 0.40 | 1.06 | 0.3270 | |
| C2 | 1.13 | 1 | 1.13 | 2.98 | 0.1148 | |
| Residual | 3.80 | 10 | 0.38 | |||
| Lack of Fit | 3.15 | 5 | 0.63 | 4.81 | 0.0550 | not significant |
| Pure Error | 0.66 | 5 | 0.13 | |||
| Cor Total | 400.40 | 19 |
| Polymer (mg.mL) | Lipid (mg.mL) | PVA (%) | Size (nm) | EE (%) | Desirability | |
|---|---|---|---|---|---|---|
| predicted formulation | 4.8 | 3 | 3 | 95.77 | 87.73 | 0.94 |
| Actual Optimized formulation | 4.8 | 3 | 3 | 96.24 ± 2.62 | 83.43 ± 3.67 | …………… |
Conclusion
Melphalan was loaded onto the HLPNPs using a single-step nanoprecipitation method. The NPs prepared were evaluated in terms of size, size distribution, zeta potential, EE%, and cytotoxicity. Results indicated that the synthesized NPs had a high EE%. Moreover, the cytotoxicity effects of the loaded drug, compared to the standard drug, increased against the ovarian cancer cells. Overall, the results of this study suggest evaluating the efficacy of the nanoformulation in-vivo to confirm the usefulness of the mentioned formulation.
Conflict of interest
The authors confirm that this article’s content has no conflict of interest.
CRediT authorship contribution statement
Mrs. M mirnezami and Mr. A Heydarinasab: Writing - original draft, Conceptualization and design of the study.
Mr. A Akbarzadeh and Mr. Arjmand: Methodology, Validation, and investigation.
Mrs. M mirnezami and Mr. A Heydarinasab: Analysis of data.
Mr. A Heydarinasab and Mr. A Akbarzadeh: Writing - review and editing, Supervision.