Introduction
Polycyclic aromatic hydrocarbons (PAHs) are semi-volatile, toxic environmental and food processing contaminants of pyrolysis, pyrogenic such as incomplete combustion of gas, wood, oil, coal or other substances and organic substances (1). They have particular concern because of their potential toxic, carcinogenic, and mutagenic properties. PAHs are significantly present in food as a result of heat processes such as smoking, grilling, toasting and drying and environmental contamination (2). Food is the main source of human exposure to PAHs throughout various routes such as air, soil or water (3,4). Cereal compounds, including bread are a major component of people’s food regimen. It is a good source of energy, contains vitamins, proteins, lipids, and minerals, which are essential in the human diet (5). For example, annually in the United States, Mexico, Finland, Poland, Germany, each person consumes cereal compounds of about 20, 31, 40, 59, 81 kg, respectively (6). In Iran, bread is also a major component of people’s food regimen and based on the national nutrition and food technology research institute of Iran, the average monthly consumption of bread is 117 kg per year which is very high in comparison with other countries (7). PAHs levels of bread depend on heating processes (time and temperature), the distance from the heat source, the amount of fat in the processed food and the kind of fuel besides the kind of process (8, 9 and 10).
Reports announced that amounts of PAHs in baked bread were 2–6 times higher than the initial flour and showed a variable distribution of these contaminants at parts of crust, loaf and crumb of the final product (4). The concentration raised from 1.07–3.65 ng/g detected in raw materials to 1.59–13.6 ng/g in bread baked at different temperatures (11). It is noticeable that major factors affecting in Bread’s contamination by PAHs are the contamination of bakery raw materials, primarily flour, and the baking process (9). In general, there are no regulations for PAH in bread, and according to the European Commission, the amount of benzo[a] pyrene (BaP) and PAH4 (sum of BaP, benz[a]anthracene (BaA), benzo[b]fluoranthene (BbF), and chrysene (CHR) in processed cereal-based foods and baby foods should not go beyond 1 ng/g (12). Rozentale et al., showed the concentrations of PAHs in cereal products were in a range of 0.22-1.62 ng/g, with 14% of samples exceeding the current EU maximum permitted levels (5).
The effect of toasting bread in the formation of PAHs has been studied, and the levels of PAHs in toasted bread were found in the range of 7.38–18.0 μg/kg (13). Some researchers also showed that the average PAH levels in baked bread by various fuels such as solar, solid waste, and electricity (14).
Nowadays, different sample preparation methods have been set up for trace analysis of contaminants in various foodstuffs. QuEChERS, which stands for Quick, Easy, Cheap, Efficient, Rugged and Safe, was introduced by Anastassiades and collaborators for pesticide analysis (15). QuEChERS sample preparation, despite most analysis methods is time-saving and requires lower solvents. Also, the emerge of modern instrumental techniques like gas chromatography coupled mass spectrometer has greatly increased experts’ abilities to analyze various contaminants in complex matrices.
Sangak is a favorite Iranian traditional bread. There are a few studies concerning the occurrence of PAHs in Iranian Sangak bread samples. Therefore, the essential aim of this research was the development and validation of a GC-MS method using QuEChERS sample preparation for the determination of 10 priority PAHs in traditional Sangak bread samples. Then, the validated method was applied to the analysis of PAHs in real Sangak samples.
Experimental
Chemicals and Analytical standards of PAHs
The standard of 10 PAHs from the list of EU and EPA including; Acenaphthene (ACP), Fluorene (FLR), Phenanthrene (PHE), Anthracene (ANT), Fluoranthen (FLA), benzo[a]anthracene (B[a]A), chrysene (CHR), benzo[b]fluoranthene (B[b]F), benzo[k]fluoranthene (B[k]F), benzo[a]pyrene (B[a]P), and triphenyl phosphate (TPP) as internal standard were purchased from Sigma Aldrich /Fluka/Riedel-de-Haën (United States). All solvents including Ethyl acetate, acetonitrile and methanol (analytical grade for residue analysis, HPLC gradient grade) were purchased from Merck, anhydrous magnesium sulfate, sodium acetate and Bondesil-primary secondary amine (PSA, 40 μm) were supplied by Sigma Aldrich /Fluka/Riedel-de-Haën (United states). Deionized water was obtained from a Millipore Milli-Q water purification system.
Standard Preparation
All of the PAH compounds (Table 1) stock solutions were prepared by weighing exactly 10 mg of each standard, dissolved separately in 10 mL of ethyl acetate. A mixed intermediate standard solution at 5ng/g was prepared via the appropriate dilution of the stock solutions in acetonitrile (ACN). This solution was used as the spiking solution for the validation experiments. A stock solution of TPP in EtAc at a concentration of 20 ng/g was used as the internal standard, and an aliquot (50 μL) of this solution (20 μg/mL) was added to the spiked bread sample. All standard solutions were prepared in an amber color volumetric flask (to avoid light exposure) and stored at -20˚C whenever not in use.
Sample Preparation
The QuEChERS method developed for the analysis of pesticides by Anastassiades et al. was used with some modifications to analyze PAHs in bread (15). Each sample was carefully ground and then, 5 g of each homogenized sample was accurately weighed and placed in a 50 mL centrifuge tube. Appropriate concentrations of the mixed working standard solution (for spiking) and 50 μL of the internal standard solution (200 ng/mL) were added to the tube, and 14 mLof ACN was added. The mixture was vortex mixed for 3.0 min, followed by adding a mixture of 2 g anhydrous MgSO4 and 1.5 g sodium acetate and subjected to vortex mixing for 3.0 min again. The mixture was centrifuged (Hettich, universal 320r from Germany) for 5 min at 9056 g, and 7 mL of the supernatant was then transferred into an appropriate tube placed in a nitrogen evaporator and dehydrated until dry. The residue was reconstituted in 0.5 mL ACN. The mixture was mixed for 3.0 min, followed by sonication for 3.0 min. Then the solution was transferred to a tube containing 60 mg anhydrous MgSO4 and 20 mg PSA. The mixture was vortex mixed vigorously for 1 min and centrifuged for 5 min at 18894 ×g. Finally, a 0.5 mL aliquot of the cleaned extract was transferred into an amber tube, and 1.0 μL was injected into GC-MS.
GC-MS Analysis
Gas chromatography (Model 7890 A, Agilent Technologies, USA) was employed using a mass spectrometry detector (Model 5975 C, Agilent technologies, USA) equipped with a split/splitless injector and an Agilent auto-sampler. A DB-5MS Agilent capillary column (30 m × 0.25 mm I.D., 0.25 μm film thicknesses) was used along with the following oven temperature program: initial temperature 80 °C, held for 2 min, 20 °C/min ramped to 140 °C, followed by 5 °C/min ramped to 315 °C. Helium (99.999%) was used as the carrier gas at a constant flow rate of 1 mL/min. The injection port, quadrupole mass analyzer, transfer line and ion source was adjusted at 300 °C, 100 °C, 280 °C, 230 °C, respectively, and the splitless mode was used. After acquiring the ion chromatogram in selected ion monitoring (SIM) mode, peaks were identified by their retention time and mass spectra. At least three ions were applied to the detection and determination of chemicals. The most abundant ion that had the highest signal-to-noise ratio and showed no evidence of chromatographic interference was selected for quantification.
Method validation
Method efficiency is a substantial agent for all laboratories to guarantee the validity of the routine analysis. The method was tested to assess for matrix effect, linearity, limits of detection (LOD) and quantitation (LOQ), recovery and precision. Linearity was studied using the spiked calibrations method by analyzing six concentration levels using triplicate analysis over the concentration range of 10-500 ng/g. The spiked calibration curves (six points) for all the analytes were attained by plotting the ratio of peak area of each compound to that of internal standard against the concentration of the corresponding analyte. Recovery data were used to assess accuracy while their RSD was the day-to-day precision. To determine the mean recoveries and precision (repeatability) expressed as the coefficient percent of the variation, three spiked blank bread samples at concentration levels of 25, 50 and 200 ng/g were prepared and treated according to the procedure described in the sample preparation in triplicate. This was performed for three consecutive days. An aliquot of 50 μL of triphenylphosphate (TPP) solution in acetonitrile (20,000 ng/mL) was added to the spiked bread sample as an internal standard. The LOD and LOQ levels were calculated to evaluate PAH compound concentrations resulting in a signal-to-noise ratio of 3 and 10, respectively. Sample components impact on matrix effect by interfering gas chromatography and/or mass spectrometry (18). Bread sample, including salt, wheat protein and carbohydrates is no exception and can suppress or enhance targeted ions in mass spectrometry assay. Matrix effect (ME) was estimated by comparing the slopes of spiked calibration curves (A) and standard curves dissolved in extraction solvent (B) allowed the estimation of the recovery and matrix effect in terms of ion (19). The matrix effect was calculated as:
ME (%) = (1 - A/B) × 100
Determination of PAHs in Sangak samples
Forty-seven traditional Sangak bread samples were provided based on the national standard of Iran (20) collected from Sangak bakeries located in Tehran city. Traditional bread was prepared by direct and indirect heating of natural gas at 170-300 °C. After collection, all samples were covered with aluminum foil and transported to the lab. Each sample was coded and dried to lose its moisture within one day. Then all of the samples were ground and stored in amber glass bottles at −20 °C until analysis. Finally, a 5.0 g portion of homogenized samples was weighted and analyzed.
Results and Discussion
Determination of gas chromatography-mass spectrometry
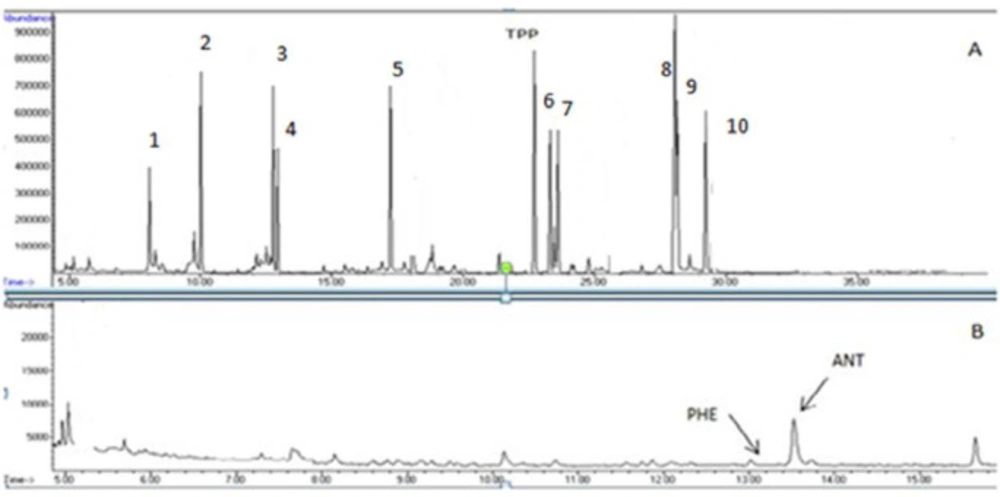
For analysis of the studied PAHs, optimization of GC and MS conditions was accurately conducted. Continuous injections obtained various GC parameters, including; MS conditions, oven temperature program, and define to a suitable column. Therefore, the SIM mode was employed for analysis of the studied chemicals. After acquiring mass data, one ion with higher intensity was employed as a quantification ion. For confirmation, at least two ions with the selected ion and their ion ratio and retention times were applied. Table 1 shows molar masses, SIM conditions, retention times, and related ion ratios for the studied PAHs. In addition, a chromatogram of the mixed standard related to 10 PAHs (A) also the same PAHs spiked in Sangak sample (B) was shown in Figure 1.
Method validation
Matrix effects
Due to complexity, matrix effect (ME) is considered as the main challenge in food contaminant analysis. Matrix effect by signal enhancing or suppressing could affect the obtained results in analytical procedures. Hence it is necessary to examine this effect very well. There are various ways for studding the matrix effect. In this study, a solvent-based and spiked calibration curves approach was employed. Calibration standards are prepared by adding standard solution to blank bread samples subjected to the same sample preparation procedure. The calibration curve is constructed by analyzing in triplicate six concentration levels (10, 25, 50, 100, 200 and 500 ng/g). As shown in Table 2, the matrix effect for all of the analytes presents a strong signal enhance (>50%) except the matrix effect for BbF considered medium signal enhance whereas that was medium suppression signal in BaA. Therefore, spiked calibration curves were used to overcome the matrix effect, in this research.
Linearity and linear range
Spiked calibration standards at levels of 10, 25, 50, 100, 200 and 500 ng/g were prepared by the addition of 10, 25, 50, 100, 200 and 500 μL of standard stock solutions with a concentration of 5000 ng/mL to 5 g of blank bread samples in each case respectively. Quantification of the PAH compounds in bread samples was performed by using an internal standard method. Therefore 50 μL of TPP solution in acetonitrile (20,000 ng/mL) was added to all spiked bread samples. Calibration curves showed a linear relationship between the concentration and peak area ratios linear in the concentration range of 10-500 ng/g with a coefficient of determination (R2) ranging between 0.994 and 0.999. It shows that the extraction process and analytical method after validation have enough efficiency to determine PAHs at trace levels (Table 3).
LOD and LOQ measurement
Limits of detection (LODs) and quantification (LOQs) were calculated based on the signal-to-noise ratio of equal to 3 and 10, respectively. The LODs and LOQs, as shown in Table 3, were between 0.139-0.785 ng/g and 0.459-2.591 ng/g, respectively.
Recovery and precision
The mean extraction recoveries were determined by applying the full procedure to triplicate samples in three consecutive days of analysis at three spiking levels, including 25, 50 and 200 ng/g with the same operator and laboratory. The percentage of mean recoveries obtained 92-106.0% for each PAH compound is considered an acceptable range of European Commissions, regulation (European Commi-ssions). Precision was expressed as relative standard deviation (RSDr) and evaluated like the recovery in different days. The average relative standard deviations (RSDs) of PAHs in bread was in the range of 3.16-7.78% with a satisfactory precision (RSDr < 20%) which were in the acceptable range of European guidelines (16, 17). the values of mean recovery and RSD percentage for each spiking level are presented in Table 4.
Determination of PAH compounds in bread samples
As the results of the analysis of bread samples shown in Table 5, 47 Sangak samples were analyzed to evaluate the practicality of the method. The results showed that the PHE compound was detected in 6 samples (12.76%) at the mean level of 5.57 ± 5.21 which three samples were between LOD and LOQ and the 3 other samples were higher than LOQ. It is noticeable that all of the positive samples were detected in provided bread by undirected heating. On the other hand, 3 (6.38%) of ANT compound at the mean level of 14.59 ± 5.52 was detected in directed heating samples that all of them were higher than the amount of LOQ.
Overall, 9 (19.4%) out of 47 Sangak bread samples were contaminated with PHE and ANT compounds at the mean level of 10.08 ± 6.38 ng/g which was higher than the permissible limit of European Commission regulatory control value for BaP (1 ng/g of wet weight) in processed cereal-based foods and baby foods for infants and young children (20).
Previous studies have shown that there are few published papers in the field of PAHs in Iranian bread. Only research around the traditional bread (Sangak) was reported by Eslamizad et al. they developed a method for investigating BaP in Sangak bread (21). They analyzed 29 Sangak bread samples from Tehran’s bread bakeries in 2014. Results showed that two Sangak samples were contaminated with BaP. Their another study showed that 35.5% and 13% of Sangak bread samples collected in Tehran and Shiraz were contaminated with BaP respectively (22). In current study, besides of BaP, nine other compounds were analysed, but BaP was not detected in any samples. Research of Al-Rashdan et al on Seven Iranian bread samples showed NPH, FLR, and PHE were the most three abundant chemicals found in the studied breads (23). These findings were in accordance with Rashdan et al. studies.
| NO. | Compound | Abbreviation | Molar | Quantification Ions (m/z) | Confirmation Ions (m/z) | Ion Ratio | Retention Time (min) |
|---|---|---|---|---|---|---|---|
| 1 | Acenaphtylene | ACL | 152 | 152 | 152,151,76 | 4.509 | 8.18 |
| 2 | Fluorene | FLR | 166 | 166 | 166,165,82 | 1.176 | 10.16 |
| 3 | Phenanthrene | PHE | 178 | 178 | 178,176, 152 | 5.13 | 12.91 |
| 4 | Anthracene | ANT | 178 | 178 | 178,176, 152 | 8.441 | 13.09 |
| 5 | Fluoranthene | FLA | 202 | 202 | 202, 203, 201 | 6.76 | 17.40 |
| 6 | Benz(a) anthracene | B(a)A | 228 | 228 | 228.226,229 | 2.906 | 23.66 |
| 7 | Chrysene | CHR | 228 | 228 | 228.226,229 | 3.456 | 23.79 |
| 8 | Benzo(b)fluoranthene | B(b)F | 252 | 252 | 252, 253, 250 | 5.106 | 28.25 |
| 9 | Benzo(k)fluoranthene | B(k)F | 252 | 252 | 252, 253, 250 | 3.431 | 28.35 |
| 10 | Benzo(a) pyrene | B(a)P | 252 | 252 | 252, 253, 250 | 6.624 | 29.76 |
| NO. | Compound | Abbreviation | Spiked calibration curve | Solvent calibration curve | A | Matrix Effect (%) | ||
|---|---|---|---|---|---|---|---|---|
| Slope | R2 | Slope | R2 | |||||
| 1 | Acenaphtylene | ACL | 0.002 | 0.998 | 0.023 | 0.997 | 0.098 | 90.15 |
| 2 | Fluorene | FLR | 0.005 | 0.997 | 0.020 | 0.999 | 0.249 | 75.12 |
| 3 | Phenanthrene | PHE | 0.005 | 0.998 | 0.010 | 0.994 | 0.490 | 50.94 |
| 4 | Anthracene | ANT | 0.003 | 0.998 | 0.008 | 0.999 | 0.410 | 58.98 |
| 5 | Fluoranthene | FLA | 0.007 | 0.999 | 0.018 | 0.994 | 0.379 | 62.13 |
| 6 | Benz(a) anthracene | B(a)A | 0.005 | 0.999 | 0.004 | 0.999 | 1.366 | -36.64 |
| 7 | Chrysene | CHR | 0.006 | 0.999 | 0.013 | 0.998 | 0.423 | 57.65 |
| 8 | Benzo(b)fluoranthene | B(b)F | 0.008 | 0.999 | 0.012 | 0.999 | 0.705 | 29.52 |
| 9 | Benzo(k)fluoranthene | B(k)F | 0.003 | 0.999 | 0.009 | 0.999 | 0.341 | 65.91 |
| 10 | Benzo(a) pyrene | B(a)P | 0.006 | 0.992 | 0.016 | 0.995 | 0.393 | 60.71 |
| No. | Compound | Regression Equation | Determination of Coefficient (R2) | LODa | LOQb |
|---|---|---|---|---|---|
| 1 | Acenaphtylene | y = 0.002x + 0.038 | 0.998 | 0.651 | 2.150 |
| 2 | Fluorene | y = 0.005x + 0.052 | 0.997 | 0.752 | 2.483 |
| 3 | Phenanthrene | y = 0.005x + 0.077 | 0.998 | 0.611 | 2.015 |
| 4 | Anthracene | y = 0.003x + 0.011 | 0.998 | 0.561 | 1.852 |
| 5 | Fluoranthene | y = 0.006x - 0.006 | 0.999 | 0.139 | 0.459 |
| 6 | Benz(a) anthracene | y = 0.005x + 0.012 | 0.999 | 0.243 | 0.803 |
| 7 | Chrysene | y = 0.013x - 0.068 | 0.998 | 0.204 | 0.675 |
| 8 | Benzo(b)fluoranthene | y = 0.008x + 0.015 | 0.999 | 0.349 | 1.152 |
| 9 | Benzo(k)fluoranthene | y = 0.003x + 0.008 | 0.999 | 0.389 | 1.285 |
| 10 | Benzo(a) pyrene | y = 0.017x - 0.106 | 0.994 | 0.785 | 2.591 |
| No. | Compounds | 25 (ng/g) | 50 (ng/g) | 200 (ng/g) | Average recovery | Average | |||
|---|---|---|---|---|---|---|---|---|---|
| Recovery | RSDr | Recovery | RSDr | Recovery | RSDr | ||||
| 1 | ACL | 98.21 | 5.66 | 111.72 | 6.89 | 99.18 | 10.81 | 103.04 | 7.78 |
| 2 | FLR | 90.37 | 3.36 | 92.15 | 6.24 | 97.70 | 5.62 | 93.41 | 5.07 |
| 3 | PHE | 89.19 | 1.39 | 96.83 | 5.09 | 110.04 | 3.00 | 98.69 | 3.16 |
| 4 | ANT | 93.96 | 1.37 | 98.26 | 3.78 | 111.89 | 5.98 | 101.37 | 3.71 |
| 5 | FLA | 108.52 | 1.11 | 107.89 | 2.31 | 102.26 | 8.22 | 106.22 | 3.88 |
| 6 | B(a)A | 86.75 | 3.58 | 94.43 | 2.30 | 98.42 | 8.89 | 93.19 | 4.92 |
| 7 | CHR | 100.14 | 1.67 | 99.95 | 1.89 | 101.79 | 6.97 | 100.62 | 3.51 |
| 8 | B(b)F | 93.35 | 1.93 | 104.36 | 2.74 | 101.38 | 5.93 | 99.69 | 3.53 |
| 9 | B(k)F | 90.31 | 2.44 | 98.06 | 2.56 | 109.42 | 12.63 | 99.27 | 5.87 |
| 10 | B(a)P | 90.28 | 3.09 | 94.18 | 4.40 | 93.79 | 5.62 | 92.75 | 4.36 |
| No. | Compounds | No. of positive samples | Mean ± SD | Min Level | Max Level | No. of samples in the range ng/g | |
|---|---|---|---|---|---|---|---|
| LOQ-LOQ | > LOQ | ||||||
| 1 | ACL | 0 | 0 | nd | nd | 0 | 0 |
| 2 | FLR | 0 | 0 | nd | nd | 0 | 0 |
| 3 | PHE | 6 (12.76%) | 5.57 ± 5.21 | 0.64 | 11.06 | 3 | 3 |
| 4 | ANT | 3(6.38%) | 14.59 ± 5.52 | 10.12 | 20.77 | 0 | 3 |
| 5 | FLA | 0 | 0 | nd | nd | 0 | 0 |
| 6 | B(a)A | 0 | 0 | nd | nd | 0 | 0 |
| 7 | CHR | 0 | 0 | nd | nd | 0 | 0 |
| 8 | B(b)F | 0 | 0 | nd | nd | 0 | 0 |
| 9 | B(k)F | 0 | 0 | nd | nd | 0 | 0 |
| 10 | B(a)P | 0 | 0 | nd | nd | 0 | 0 |
| ∑10 PAHs | 9 (19.14%) | 10.08 ± 6.38 | 0.64 | 20.77 | 3 | 6 | |
Conclusion
In the present study, the QuEChERS procedure coupled with gas chromatography-mass spectrometry was used to extract and determine ten polycyclic aromatic hydrocarbons in bread samples. This method is advantageous in terms of total solvent utilization and also the extraction solvent is less toxic. After optimization of the parameters affecting the analysis efficiency, the identification and quantification of the PAHs were carried out by applying the mentioned techniques for the analysis of bread samples. The results showed that applied method results in a satisfactory correlation coefficient, recovery and precision. Moreover, the use of spiked calibration curves for constructing the calibration curve substantially reduced adverse matrix-related effects. The results reveal that only 2 compounds of 10 analyzed PAHs were detected in traditional bread (Sangak) at the level of higher than maximum levels set for processed cereal-based foods and baby foods.