Introduction
The patients who have undergone heart valve replacement need warfarin, an anticoagulant, for the rest of their lives (1). However, maintaining the therapeutic doses of warfarin is still problematic especially in the initial stage of warfarin therapy, because of its narrow therapeutic index and large inter-individual variability in the patient’s response. A sub-therapeutic dose might not prevent clot formation and stroke, while a high dose might leads to bleeding (2). Thus, the appropriate dose of warfarin must be personalized for each patient. The criterion used for response to this drug is the prothrombin ratio (PR) expressed as international normalized ratio (INR) and measured by the clotting tendency of blood (3). Warfarin dose requirement is determined by a number of different factors, with VKORC1 genotype being the major constituent (by 33%) while CYP2C9 genotype (by 22%) together with age, weight, and gender (by 12%) also contribute (4, 5).
Vitamin K epoxide reductase complex (VKORC1) which encodes the VKOR enzyme is located on chromosome 16 p 11.2 (6). The VKOR enzyme is essential for the coagulation process and its inhibition by warfarin leads to perturbation of blood coagulation (7). The genetic polymorphism -1639 G>A is located in the promoter region of the VKORC1 gene at a potential E box (CA/GNNTG). It has been shown that this position is an important element for mediating the transcription of VKORC1 and that it will change the promoter activity when adenine substitutes for guanine at the position (8). Due to the -1639 G>A polymorphism, the qualitative and quantitative expression of VKORC1 and subsequently of warfarin dose requirement could be significantly influenced (9, 10).
Warfarin is a racemic mixture of S- and R- isomers of which S-warfarin has considerably more anticoagulant activity (11). The CYP2C9 gene located on the forward direction of chromosome 10, encodes enzyme which involves in the clearance of various therapeutic agents including S-warfarin (12). The allelic variants CYP2C9 *2 (rs 1799853, c. 430 C>T, p. Arg144Cys) and *3 (rs 1057910, c. 1075 A>C, p. Ile359Leu), are most common functionally significant coding SNPs that affect enzyme activities and cause impaired metabolic capacity of CYP2C9 enzyme. The patients with one or two of these variants require lower dose of warfarin and encounter higher risk of bleeding compared with the patients carrying wild type (*1) allele (13).
Different allelic frequencies have been shown for VKORC1 -1639 G>A and CYP2C9 variants in various ethnic groups. Iran is a country with different ethnic populations including Turks, Arabs, Baloches, and Kurds, which may have different genetic, social and environmental backgrounds. However, there are limited data on the genetic polymorphisms related to warfarin metabolism and action for the Iranian population (14). Hence, this study for the first time aimed to assess the effect of VKORC1 -1639 G>A and CYP2C9 variants together with demographical characteristics of patients on warfarin doses requirement among patients mostly with heart valve replacement in West Azerbaijan, Iran.
Experimental
Subjects and Criteria
This study was performed between February 2016 and March 2017 on a total of 185 patients aged 25-86 mostly with heart valve replacement. The patients who received a maintenance dose less than 1.5 mg/day, were considered as sensitive to warfarin. Patients on more than 7.5 mg/day were considered as warfarin resistant. The patients with intermediate dosage of warfarin (1.5 to 7.5 mg/day) were considered as control population (15). The patients who had stable doses and international normalized ratio (INRs) between the ranges of 2.5- 3.5 within the last 4 weeks were included in the study. The exclusion criteria were suffering from cancer, liver, and kidney diseases. All procedures performed in this study were approved by the Scientific and Ethical Committee of Urmia University of Medical Sciences (Ir.umsu.rec.1394.282).
A volume of 2 mL of peripheral blood obtained from each patient was transferred to EDTA- containing tubes in order to prevent clotting and stored at -20 °C prior to the experiments. The prothrombin time- international normalized ratio (PT-INR) test was used to determine the clotting tendency of the blood samples in the measurement of warfarin dosage. Questionnaires were designed for the demographic characteristics of the patients including sex, age, weight, height, ethnicity, type of heart valve, diabetes, blood pressure, and smoking.
Genotyping of VKORC1 and CYP2C9 alleles
Genomic DNA was extracted from individual blood samples employing DNA extraction mini kit (YTA Company, Iran) according to the manufacturer’s instructions. To detect polymorphism -1639 G>A in VKORC1 a polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) analysis was carried out according to Sconce et al. (2005) (3). To detect CYP2C9 *2 and CYP2C9 *3 polymorphic alleles, a polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) analysis was performed as described by Daly et al. (2006) (16).
Statistical analysis
Mean ± standard deviation (SD) along with median interquartile range (IQR) of warfarin doses were calculated in demographic, biologic, and clinical variables groups. The Pearson correlation test was used to examine the association of age, Height, weight, and BMI with warfarin dose. Significance differences in daily maintenance dose of warfarin between different genotype groups were evaluated by independent samples t-test and one-way analysis of variance (ANOVA). The normality assumption of warfarin dose was evaluated by Shapiro-Wilk test. If needed the square root transformation was done to normalize warfarin dose distribution and calculate the correct p-value in comparisons. The genotype distributions for the studied SNPs were calculated and checked to be in Hardy-Weinberg equilibrium (HWE) using Chi-square goodness-of-fit test with Degrees of freedom (df) of one. Box plot analysis was performed to graphically display the dispersion and skewness of warfarin dose among the genotype groups. The backward stepwise linear regression analysis was used to model the relationships of the patient’s demographic variables and genotypes with daily warfarin dose requirements. At the first step of backward regression method all of the studied variables including; age (years), ethnicity (Turk/others), hypertension, VKORC1 and CYP2C9 genotypes, BMI (kg/m2), sex (female/male) and smoking were included in the model. At the next steps the variables with weakest none-significant effect were excluded and ultimately a novel warfarin dosing algorithm was presented. All of calculations and statistical analysis were done by IMB SPSS Statistics 23 (IBM SPSS Inc. Chicago, IL). The statistical level of significance was set at P < 0.05.
Results
Among 200 patients mostly with heart valve replacement, 185 individuals (112 females and 73 males; mean age 54.7 ± 14) who met the inclusion criteria were included in the study. Distribution of the daily dosage of warfarin was 0.28 to 12.10 mg/day among all the patients while the mean daily dose of warfarin was 4.26 ± 2.43 mg/day. The descriptive statistics of the patients’ demographic, biologic, and clinical variables are shown in Table 1.
The VKORC1 (rs 9923231, -1639 G>A) polymorphism, genotype frequencies were 20.5%, 40.5%, and 38.9% for GG, GA, and AA genotypes, respectively. The allelic frequency for VKORC1 -1639 A was 60%. In the studied population the CYP2C9 *2 mutant allele frequency was found to be 12.6%. Out of 170 patients, 39 were heterozygous (CT) (22.9%) and 2 were homozygous (TT) (1.2%). The CYP2C9 *3 mutant allele frequency was found to be 25.8%. Out of 170 individuals, 82 were heterozygous (AC) (48.2%) and 3 were homozygous (CC) (1.8%). Overall genotype frequency for CYP2C9 was determined to be CYP2C9 *1/*1 (35.7%), *1/*2 (16.2%), *1/*3 (35.1%), *2/*2 (1.6%), *2/*3 (9.2%), and *3/*3 (2.2%). No significant deviation from Hardy Weinberg equilibrium was observed for CYP2C9 variants (P > 0.05).
The results of the mean comparison among the studied patients’ cohort revealed that the mean of warfarin dose had not significant difference with sex, heart valve type, smoke, diabetes, and hypertension (P > 0.05). The patients who consumed alcohol with the mean warfarin dose of 6.39 ± 2.45 mg/day (vs. non-alcohol consumers) had significantly higher and the Turkish patients with the mean warfarin dose of 4.16 ± 2.52 mg/day (vs. other ethnicities) had significantly lower warfarin dose requirement (P > 0.05). The Pearson correlation result showed significantly negative association between age and warfarin dose (r = -0.371, P < 0.001) (Table 2).
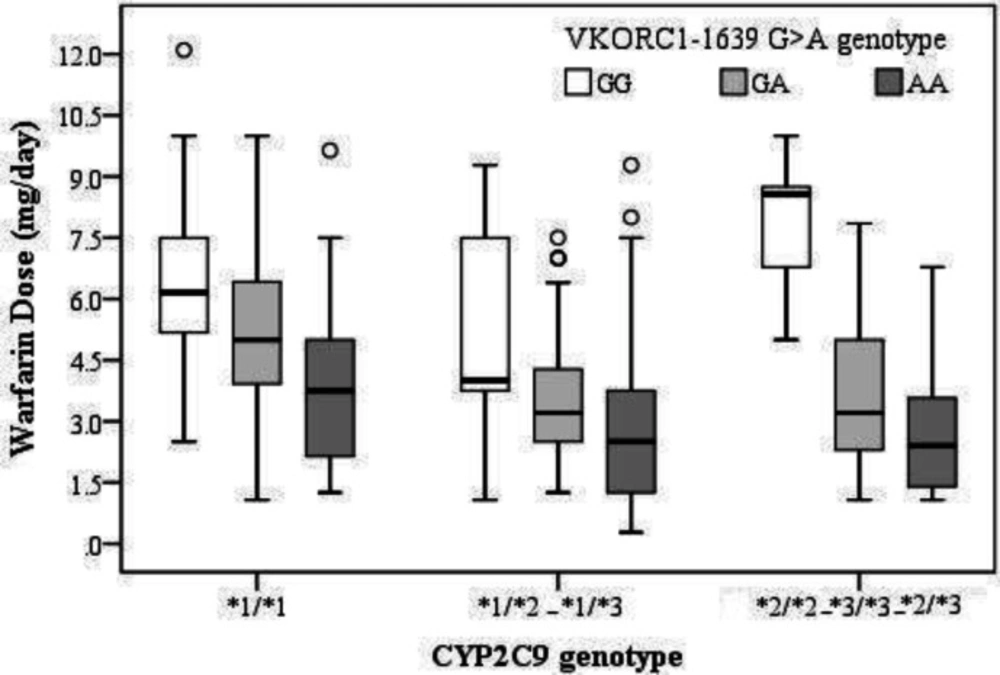
Comparison of the mean warfarin doses requirements among VKORC1 -1639 genotypes revealed significant differences between the genotypes. Mean warfarin dose requirement was obtained 6.13 ± 2.59 mg/day for people with genotype GG, 4.15 ± 1.92 mg/day for genotype GA and 3.37 ± 2.29 mg/day for the genotype AA. Pairwise comparisons of mean warfarin dose requirements among the patients with GG, GA, and AA genotypes indicated significant differences (P < 0.001 for GG vs GA and GG vs AA, P = 0.02 for GA vs. AA). Also, the mean of warfarin dose requirements significantly differed among CYP2C9 genotypes. Pairwise comparisons of warfarin dose requirements across the patients with genotypes of *1/*1 (5.10 ± 2.40 mg/day), *1/*2, *1/*3 (3.65 ± 2.18 mg/day), and *2/*2, *2/*3, *3/*3 patients (4.32 ± 2.82 mg/day) indicated that the mean warfarin dose requirement of the people with genotype *1/*1 significantly differed from that of the studied cohort with the genotype *1/*2, *1/*3 (P < 0.001). However, the comparisons among *1/*1 vs. *2/*2, *2/*3, *3/*3 (P = 0.22) and*1/*2, *1/*3 vs.*2/*2, *2/*3, *3/*3 (P = 0.54) showed no significant differences (Table 3). The simultaneous effect of the variant alleles of VKORC1 -1639 G>A and CYP2C9 is shown in Figure 1.
Final regression model results of warfarin dose requirement showed significant effects of age, hypertension, and genotype on warfarin dose (P < 0.001) (Table 4). The negative impact of age was significant on warfarin dose (β = -0.02, P < 0.001). Hypertensive patients had a higher warfarin dose requirement (β = 0.27, P < 0.001). The examination of the VKORC1 -1639 G>A genotype effect indicates that the patients with AA (β = -0.62, P < 0.001) and GA genotypes (β =-0.43, P = 0.001) had lower warfarin dose requirement compared to GG genotype. The patients with *1/*2, *1/*3 (β = -0.34, P < 0.001) and *2/*3, *2/*3 (β = -0.28, P = 0.017) of CYP2C9 genotype had lower warfarin dose requirement compared to *1/*1 genotype. The stepwise evaluation of the effect of the patients’ demographic, biologic, and clinical variables and genotypes indicated that the order of variables elimination from regression models, according to weakest impact, were as follows: sex, smoking, alcohol, diabetes, ethnicity, BMI and in the final step the valve type was excluded from the model.
Discussion
Optimizing the appropriate dose of warfarin for each individual based on genetic and environmental factors, especially in the initial stage of its usage, will lead to a reduction in the side effects of this drug and in turn decreased healthcare costs for the patients and hospitals (2, 17). The aim of the current study was to investigate the effect of -1639 G>A VKORC1 and CYP2C9 polymorphisms together with non-genetic factors that might contribute warfarin-dose variability, with the view of developing an individualized dosing regimenin North-western of Iran.
The association of VKORC1 polymorphism with warfarin dose requirement in this study was in agreement with what was reported in other populations such as United Kingdom (3), United States (18), Turkey (19), and China (20). The role of VKORC1 polymorphisms in warfarin maintenance dose was identified by D’ Andrea et al. (21). Yuan and colleagues in 2005 have reported the association of -1639A allele with low warfarin dose requirement (8). Rieder et al. have introduced haplotypes for VKORC1 on the basis of a panel of SNPs and concluded that some polymorphisms are informative about warfarin dose among European–Americans (22). Interestingly, while VKORC1 -1639 G>A is important in the patients who are sensitive or resistant to warfarin, different genotype frequencies were reported from seven East Asian countries (Taiwan, India, Indonesia, Philippines, Thailand, Vietnam and China). For example, AA and GG genotype frequencies were 67% and 6% in China’s population but 7% and 80% in India, respectively (23).
The association of CYP2C9 variants with warfarin dose requirement also has been reported in 1999 for the first time (24). The presence of CYP2C9 *2 and *3 alleles emphasizes the fact that a drug e.g. warfarin will be metabolized more slowly and individuals carrying these alleles will be more likely at the risk of bleeding and severe drug poisoning. Different allelic frequencies have been reported for CYP2C9 *2 and *3 in different racial groups. The allelic frequencies of CYP2C9 *2 and *3 in our study were 12.5% and 25.8%, respectively. But the frequency of these alleles has been reported 9.1% and 10%, respectively in the northwest of Iran. In the investigation conducted on 120 individuals in northeast of Iran 9.1% for CYP2C9 *2 and 10% for *3 were reported which showed lower variant allele frequencies in comparison to our research (25). Our studied region is located in northwest of Iran near to the border of Turkey and the studied subjects are comprised of mostly the Turks population. So, we compared the CYP2C9 allelic frequency results in our study with those in Turkey and observed similarity to those of Yildirim et al. (17% for *2 and 26% for *3) (5) and Ozer et al. (2010) (13% for *2 and 15% for *3) in the Turkish population (26). Our frequency result was also different from European population (12.5% CYP2C9 *2 and 8.5% CYP2C9 *3) (27).
Studies have also revealed that several factors affect the variability in warfarin dose, including age, body size, vitamin K intake, interacting medications, and genetic variants (28). This study showed that the ethnicity is an important factor in distribution of genetic polymorphisms. Previous studies also proved the role of ethnicity in CYP2C9 and VKORC1 variant frequency and in turn the amount of warfarin was required among Asian, European, and the other ethnicities. The role of age also has been investigated and the negative correlation between age and warfarin clearance was reported (5). The older patients require a lower dose of warfarin possibly because their liver mass and subsequently content of liver VKOR is decreased, so they become more warfarin sensitive. Park et al. (2013) demonstrated the relation between warfarin dosage and age, body mass index plus genetic variants (29). However, the current warfarin dosing algorithms do not incorporate genetic and environmental factors that affect warfarin-dose requirements. Knowledge of the extent to which these factors affect anticoagulation response along with genotyping methods could help in the prediction of a more individualized loading and maintenance warfarin dose for a safer anticoagulation therapy (30). In this research we have found out that age, ethnicity and type of heart disease beside genotype affect the amount of warfarin required in our patient group.
In the multi-ethnic population of Iran, genetic diversity, and different allelic and genotypic prevalence is predictable. It is essential to investigate the distribution patterns of genetic variants in different region of country to develop a proper dosing algorithm of warfarin and better understanding of the relationship between VKORC1 and CYP2C9 variants with sensitivity to warfarin. Determining the genotypes and changes which may influence enzyme expression allows us to truly personalize warfarin dosing.
| Categorical Variables | Categories | n | % |
|---|---|---|---|
| Sex | Male | 73 | 39.5 |
| Female | 112 | 60.5 | |
| Heart Valve Type | AVR | 41 | 25.6 |
| MVR | 57 | 35.6 | |
| AVR MVR | 42 | 26.3 | |
| AF | 13 | 8.1 | |
| Other | 7 | 4.4 | |
| Smoke | Positive | 17 | 9.2 |
| Negative | 168 | 90.8 | |
| Alcohol | Positive | 6 | 3.2 |
| Negative | 179 | 96.8 | |
| Diabetes | Positive | 22 | 11.9 |
| Negative | 163 | 88.1 | |
| Hypertension | Positive | 57 | 30.8 |
| Negative | 128 | 69.2 | |
| Ethnicity | Turk | 157 | 84.9 |
| Other | 28 | 15.1 | |
| VKORC1-1639 | GG | 38 | 20.5 |
| GA | 75 | 40.5 | |
| AA | 72 | 38.9 | |
| CYP2C9 | *1/*1 | 66 | 35.7 |
| *1/*2, *1/*3 | 95 | 51.4 | |
| *2/*2, *2/*3, *3/*3 | 24 | 13.0 | |
| Warfarin Dose | 0-1.5 | 30 | 16.2 |
| 1.6-7.4 | 125 | 67.6 | |
| 7.5-12 | 30 | 16.2 | |
| Continuous Variables | Mean ± SD | Range | |
| Age (year) | 54.72 ± 14.04 | 20 – 84 | |
| Height (cm) | 72.65 ± 14.80 | 41 – 123 | |
| Weight (kg) | 163.22 ± 10.19 | 120 – 186 | |
| BMI (kg/m2) | 27.28 ± 5.36 | 14.53 – 48.59 | |
| Warfarin dose (mg/day) | 4.25 ± 2.43 | 0.28 – 12.10 |
| Categorical Variables | Categories | Mean ± SD | Median (IQR) | P-value |
|---|---|---|---|---|
| Gender | Male | 4.01 ± 2.31 | 3.57 (2.50) | 0.32 |
| Female | 4.42 ± 2.50 | 3.92 (3.91) | ||
| Valve Type | AVR | 4.99 ± 2.77 | 3.90 (4.23) | 0.18 |
| MVR | 4.00 ± 2.04 | 3.75 (2.68) | ||
| AVR MVR | 4.90 ± 2.55 | 5.00 (4.13) | ||
| AF | 3.90 ± 2.04 | 3.90 (3.21) | ||
| Other | 3.70 ± 2.06 | 3.57 (2.70) | ||
| Smoke | positive | 4.34 ± 2.74 | 3.21 (3.80) | 0.88 |
| negative | 4.25 ± 2.40 | 3.75 (3.12) | ||
| Alcohol | positive | 6.39 ± 2.45 | 6.68 (4.66) | 0.03 |
| negative | 4.18 ± 2.40 | 3.70 (2.85) | ||
| Diabetes | positive | 5.23 ± 2.97 | 4.64 (4.63) | 0.07 |
| negative | 4.12 ± 2.32 | 3.70 (2.85) | ||
| Hypertension | positive | 4.61 ± 2.68 | 4.28 (4.24) | 0.23 |
| negative | 4.10 ± 2.30 | 3.57 (2.85) | ||
| Race | Turk | 4.16 ± 2.52 | 3.57 (3.02) | 0.03 |
| Other | 4.78 ± 1.77 | 5.00 (3.08) | ||
| Continuous Variables | R | P-value | ||
| Age (year) | -0.371 | <0.001 | ||
| Height (cm) | -0.012 | 0.88 | ||
| Weight (kg) | -0.086 | 0.30 | ||
| BMI (kg/m2) | 0.089 | 0.29 | ||
| Categorical Variables | Categories | Mean ± SD | Median (IQR) | P-value | |
|---|---|---|---|---|---|
| VKORC1-1639 | GG | a6.13 ± 2.59 | 5.53 (4.40) | <0.001 | |
| GA | b4.15 ± 1.92 | 3.57 (2.50) | |||
| AA | c3.37 ± 2.29 | 2.50 (3.40) | |||
| CYP2C9 | *1/*1 | a5.10 ± 2.40 | 5.00 (3.68) | 0.001 | |
| *1/*2- *1/*3 | b3.65 ± 2.18 | 3.21 (2.70) | |||
| *2/*2- *3/*3- *2/*3 | ab4.32 ± 2.82 | 3.57 (5.32) | |||
| independent variables | Categories | Beta | Std. error | P-value | P-value |
|---|---|---|---|---|---|
| Intercept | 3.42 | 0.16 | < 0.001 | < 0.001 | |
| Age | -0.016 | 0.003 | < 0.001 | < 0.001 | |
| Blood Pressure | < 0.001 | ||||
| Positive | 0.270 | 0.079 | < 0.001 | ||
| VKORC1-1639 | < 0.001 | ||||
| AA vs GG | -0.624 | 0.096 | < 0.001 | ||
| GA vs GG | -0.427 | 0.095 | 0.001 | ||
| CYP2C9 | < 0.001 | ||||
| *1/*2-*1/*3 vs. *1/*1 | -0.337 | 0.076 | <0.001 | ||
| *2/*2- *2/*3-*3/*3 vs. *1/*1 | -0.275 | 0.114 | 0.017 |
Conclusion
In conclusion, the results of the present study showed that prescribing an appropriate dosing regimen of warfarin in accordance with the patient’s demographic and pharmacogenetic data is on the benefit of the patients treated with warfarin. This study demonstrated that the patients with VKORC1 -1639 A and CYP2C9 *2, *3 alleles would require lower dose of warfarin and are mainly considered as warfarin sensitive. As carrying the variant alleles CYP2C9 and VKORC1 A allele cause sensitivity to warfarin and the prevalence of these variants is high in the study population, considering this fact before prescribing warfarin is important.