Introduction
Phthalides are a class of secondary metabolites with a wide range of pharmacological activities including inhibition of various enzymes, anti-inflammatory, anti-atherosclerosis, blood viscosity reduction, anti-angina, anti-convulsion, and antimicrobial activities (1-4). Phthalides and their derivatives including dihydro, tetrahydro, and dimeric compounds have been found in several species of the Apiaceae family (2).
Levisticum officinale, a member of the Apiaceae family, is a rich source of phthalides and their derivatives including (Z)-ligustilide, (Z)-3-butylidenephthalide, (E)-3-butylidenephthalide, 3-butylphthalide, and levistolide A (5, 6).
L. officinale grows widely in the Hezar Mountain of the Kerman Province in Iran. The root of this plant has been used for various biological effects primarily as a diuretic, as a treatment for urinary tract infection and as a spasmolytic agent (6-8).
In order to discover new and potentially bioactive constituent from L. officinale, we investigated the ethyl acetate extract of the root. In this study, we report on the isolation, structural identification, and antibacterial activity of three phthalides, including a new one.
Experimental
General experimental procedures
NMR spectra were recorded on a Bruker AVANCE III spectrometer operating at 500 MHz for 1H NMR and 125 MHz for 13C NMR. UHPLC-MS analyses were performed on a Waters Acquity UPLC system coupled to a Waters XevoTM quadrupole time-of-flight (QToF) mas spectrometer and equipped with an electrospray source with lockspray interface for accurate mass measurements. Silica gel (70-230 mesh) was used for column chromatography, and precoated silica gel F254 (20 × 20 cm) plates for TLC, both supplied by the Merck. C18-reversed phase silica gel used for column chromatography, was purchased from Sigma.
Plant material
The roots of L. officinale were collected in July 2015 from the Hezar Mountain of Kerman Province, Iran. The plant material was identified by Prof. Farideh Attar from Tehran University. A voucher specimen (46553-TUH) has been deposited in the herbarium of the Science Faculty of Tehran University, Iran.
Extraction and isolation
Air-dried, powdered roots of L. officinale (3 kg) were extracted with n-hexane (3 x 9 L) and then ethyl acetate (3 x 9 L) by maceration at room temperature. The ethyl acetate extract was concentrated by rotary evaporation, to afford 100 g of dried extract. This extract was subjected to silica gel column chromatography (230- 400 mesh, 1 kg), with a gradient of n-hexane–EtOAc and then EtOAc–MeOH as the eluent to give 16 fractions. Fraction 6 (1.8 g) was separated on a silica gel column (230-400 mesh) into five subfractions (6a-6e). Subfraction 6a (50 mg) was applied to a reverse phase silica gel column (8 g) and eluted with methanol-water as eluent, to afford compound 1 (2 mg). Purification of subfraction 6b (40 mg) on a reverse phase silica gel (7 g) column with a gradient of methanol-water as eluent led to the isolation of compound 2 (3 mg). Subfraction 6c (70 mg) was purified on a reverse phase silica (12 g) column to give compound 3 (3.5 mg).
7-methoxy-3-propylidenephthalide (1)
Brown oil; 1H NMR (CDCl3 & CD3OD, 500 MHz) δ 7.62 (1H, t, J = 8.0 Hz, H-5), 7.21 (1H, d, J = 8.0 Hz, H-6), 6.93 (1H, d, J = 8.0 Hz, H-4), 5.63 (1H, d, J = 7.5 Hz, H-8), 4.01 (3H,s, -OCH3), 2.48 (2H, m, H-9), 1.14 (3H, t, J = 7.0 Hz, H-10), 13C NMR (125 MHz, CDCl3 & CD3OD): δ 158.7 (C-7), 147.0 (C-3), 142.3 (C-3a), 111.3 (C-4), 137.2 (C-5), 111.9 (C-6), 110.6 (C-7a(, 111.8 (C-8), 56.1 (-OCH3), 19.3 (C-9), 13.9 (C-10); HRESIMS (m/z = 205.071[M + H]+, calcd 205.086) for C12H12O3.
5-hydroxybutylidene phthalide (2)
Brown oil; 1H NMR (CDCl3 & CD3OD, 500 MHz) δ 7.76 (1H, d, J = 8.0 Hz, H-7), 7.02 (1H, s), 6.97 (1H, d, J = 8.0 Hz, H-6), 5.57 (1H, t, J = 7.8 Hz, H-8), 2.43 (2H, m, H-9), 1.54 (2H, m, H-10), 0.98 (3H, t, J = 7.0 Hz H-11), 13C NMR (125 MHz, CDCl3 & CD3OD): δ 167.4 (C-1), 161.8 (C-5), 145.1 (C-3), 141.8 (C-3a), 127.3 (C-7), 118.2 (C-6), 117.0 (C-7a), 109.6 (C-8), 105.3 (C-4), 27.7 (C-9), 22.5 (C-10), 13.8 (C-11).
7-hydroxybutylidene phthalide (3)
Brown oil; 1H NMR (CDCl3 & CD3OD, 500 MHz) δ 7.60 (1H, t, J =8.0 Hz, H-5), 7.30 (1H, d, J =8.0 Hz, H-6), 6.96 (1H, d, J =8.0 Hz, H-4), 5.84 (1H, d, J =7.7 Hz, H-8) 2.39 (2H, m, H-9), 1.65 (2H, m, H-10), 0.1.01 (3H, t, J =7.0 Hz, H-11), 13C NMR (125 MHz, CDCl3 & CD3OD): δ 158.3 (C-7), 147.7 (C-3), 142.6 (C-3a), 137.0 (C-5), 116.5 (C-6), 111.4 (C-4), 110.6 (C-7a), 109.7 (C-8), 28.0 (C-9), 22.8 (C-10), 13.7 (C-11).
Antibacterial activity
Bacterial strains
In-vitro antibacterial activity of compounds was assessed against Staphylococcus aureus ATCC 25923, Enterococcus faecium (Vancomycin-resistant clinical strain) as Gram-positive bacteria and Escherichia coli ATCC 25922 and Pseudomonas aeruginosa PTCC1430 as Gram-negative bacteria. Some strains (ATCC strains and VRE) were kindly provided by Professor M.M. Feizabadi, Tehran University of Medical sciences. PTCC strain was purchased from Iranian Research Organization for Science and Technology.
Determination of MIC
Determination of the minimum inhibitory concentration (MIC) was carried out by the broth micro-dilution method as recommended by CLSI (Clinical Laboratory and Standard Institute) with minor modifications (10). The serial dilution of each compound was made in a range of concentrations of 256-0.015 μg/mL in sterile 96-well plates. The standard saline solution was prepared to get inoculant turbidity equal to 0.5 McFarland standards. The inoculants of the microbial strains were prepared from 20 h bacterial cultures that were adjusted to 0.5 McFarland standard turbidity and were further diluted (1:100) using MHB medium just before adding to the serially diluted samples. The plates were incubated for 24 h at 37 °C. MIC values were recorded as the lowest concentrations, which could inhibit the visible growth of the microorganisms. Each experiment was done in triplicate. Cefixime and chloramphenicol were used as the standard antibacterial agent.
Result and Discussion
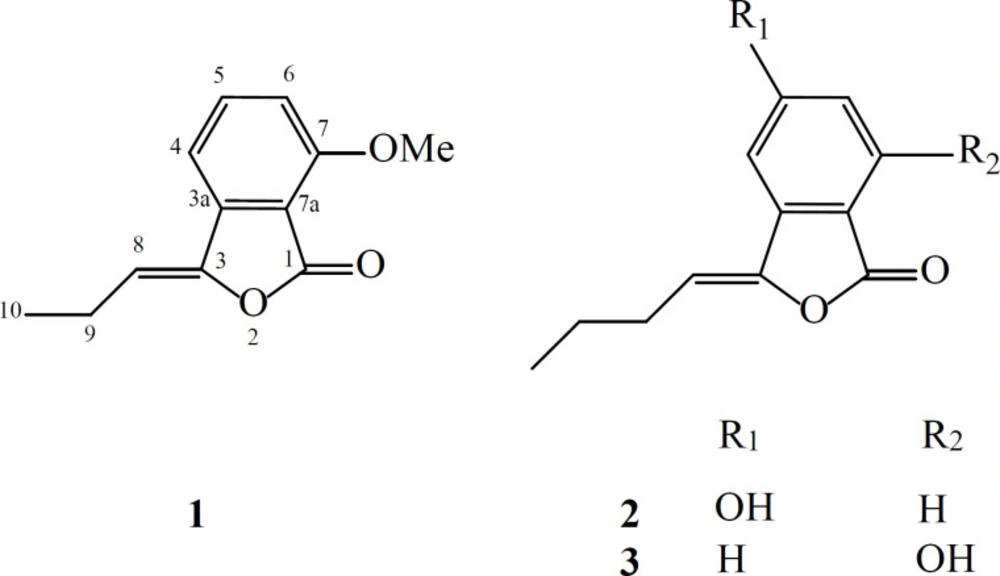
Phytochemical analysis of the ethyl acetate extract from L. officinale led to isolation and identification of a new phthalide (1), together with two knowns compounds namely, 5-hydroxybutylidene phthalide (2) and 7-hydroxybutylidene phthalide (3) (Figure 1).
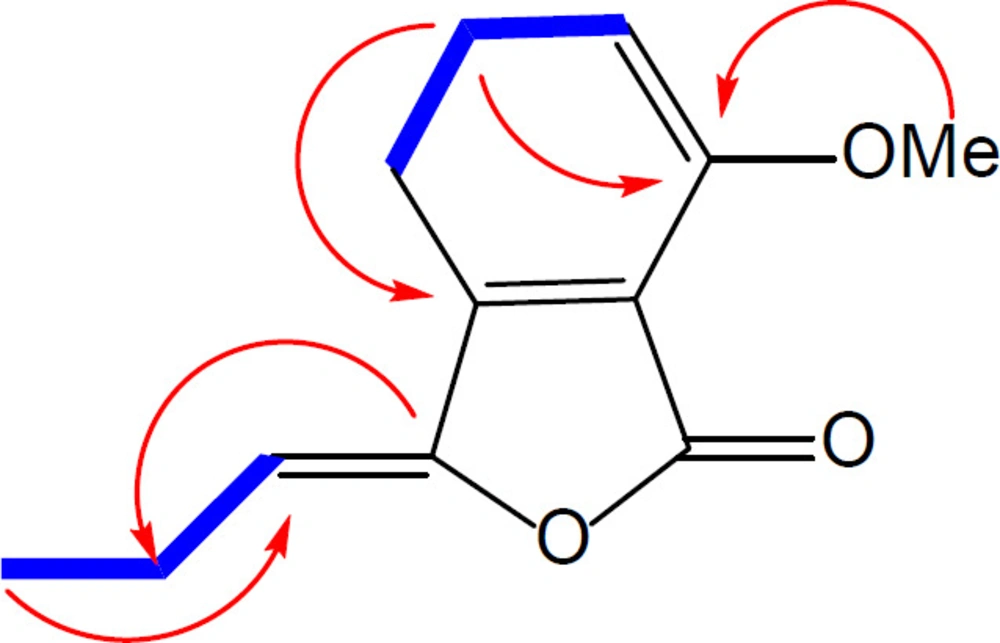
Compound 1 was obtained as a brown oil and its molecular formula, C12H12O3 was established by HR-ESI-MS (m/z = 205.071[M + H]+, calcd 205.086) with 6-degrees of unsaturation. On the basis of APT and HSQC experiments, 12 resonances in the 13C NMR spectrum (Table 1) were resolved into two methyl (corresponding to one methoxy and one aliphatic methyl), one methylene, four methine and five quaternary carbons. The 1H NMR data (Table 1) confirmed the presence of a 1, 2, 3-trisubstituted aromatic ring [δ 6.93 (1H, d, J = 8.0 Hz), 7.20 (1H, d, J = 8.0 Hz), 7.62 (1H, t, J = 8.0)]. The NMR spectroscopic data of 1 showed high similarity to those of 3, previously isolated from Petroselinum crispum [9], indicating that they are structurally related. Comparison of the 1H NMR and 13C NMR data of these two compounds indicated the loss of a CH2 group in side chain of compound 1, which was also evidenced by the 1H-1H COSY and HMBC spectral data of compound 1 (Figure 2).
Furthermore, an additional methyl signal was observed at δH/δC 4.01/56.1, indicating the replacement of the phenolic hydroxyl group by a methoxy group. This was confirmed by a HMBC correlation from the methyl group to C-7. Thus, compound 1 was ascribed the name 7-methoxy-3-propylidenephthalide.
The structure of the known compounds (2 and 3) was identified using 1H-NMR and 13C-NMR, as well as by comparison the data with those reported in literature (9).
Apiaceae plants are rich source for phthalide compounds such as dimer and simple phthalides. Among simple phthalides, butylphthalides are the most abundant, but propylphthalides are rather rare (2, 3). So, presence of compound 1 as propylphthalide in L. officinale is notable.
The activity of the isolated compounds was studied against strains of the Gram negative bacteria Escherichia coli and Pseudomonas aeruginosa and the Gram positive bacteria Staphylococcus aureus and vancomycin-resistant Enterococcus [VRE] faecium. Compound 3 showed activity against S. aureus, E. coli, and E. faecium with MIC values of 16, 64, and 128 μg/mL respectively, while compound 2 showed activity against only S. aureus and E. coli with MIC values of 128 and 256 μg/mL, respectively. Compound 1 showed reduced antibacterial activity against S. aureus and E. coli with MIC values of 256 μg/mL (Table 2). Thus, it appears that the free hydroxyl group is required for antibacterial activity in these derivatives.
Simple phthalide derivatives usually have low to moderate antibacterial activity with MIC values in the range of 4 – 0.128 mg/mL against S. aureus and E. coli (3). For example, ligustilide has previously been reported for its growth inhibitory activity against E. coli (MIC = 4 mg/mL), and S. aureus (1 mg/mL). Antibacterial activity was found for butylidenephthalide against E. coli and S. aureus with MIC values of 4 mg/mL, while (Z)‐ligustilide exhibited moderate antibacterial activity against S. aureus with MIC value of 1 mg/mL (3).
| Position | Compound 1 | |
|---|---|---|
| δC δH (J in Hz) | ||
| 1 | Not observed | - |
| Compounds | Staphylococcus aureus | Enterococcus faecium* | Escherichia coli | Pseudomonas aeruginosa |
|---|---|---|---|---|
| MIC (μg/mL) | ||||
| 1 | 256 | >256 | 256 | >256 |
| 2 | 128 | 256 | 128 | >256 |
| 3 | 16 | 128 | 64 | >256 |
| Cefixime | 2 | 16 | 4 | 64 |
| Chloramphenicol | 8 | 64 | 4 | >256 |
Conclusion
In the present study, three phthalide derivatives were isolated from the roots of L. officinale, including new one. Their structures were elucidated by means of extensive 1D, 2D NMR spectroscopy. Also, the antibacterial activity of the compounds was studied against two Gram negative and two Gram positive bacteria strains. Among them, compound 3 hold a good potential for use in future studies due to their antibacterial properties. To our knowledge, this is the first report on the isolation and structure elucidation of the chemical constituents of L. officinale in Iran. All compounds are reported for the first time from L. officinale. Simple phthalides are not widely reported as showing any significant antibacterial activity. Therefore, the antibacterial activity of compound 3 is notable.