Introduction
Breast cancer is considered as the most familiar female malignant tumor in western countries and is becoming more and more widespread in Asia (1). Currently, the survival of patients with metastatic breast cancer remains low at only 23% (2, 3). This issue prompted researchers to make a mouse model to study breast cancer and its progression as well as develop novel and more effective compounds (4, 5). Among the different models, an excellent currently available model of breast cancer is the BALB/c-derived 4T1 tumor. This tumor model shares many features with human breast cancer in terms of progressive growth in the mammary gland and active metastasis to the other organs (6).
Chemotherapy is unable to obtain clinical responses in patients with highly invasive metastatic disease (7-9). Several limitations are reported with chemotherapy using, among them, tumor drug resistance and risk of toxicities are the most important ones (10-12). Therefore, there is an essential need for more effective approaches to treatment of breast cancer (13, 14). Nonsteroidal anti-inflammatory drugs (NSAIDs) have the potential to be used as anti-cancer agents (15). In this regard, celecoxib as the first selective cyclooxygenase-2 (COX-2) inhibitor has been approved for treatment of different types of cancer, which acts through COX-2-dependent and -independent mechanism (11, 16).
Celecoxib with a 1, 2-di-aryl heterocyclic structure is an ideal lead compound for developing novel derivatives possessing potent anticancer property (17). Currently, researchers directed more attention towards compounds with tri-aryl structures as more potent chemotherapeutic or chemo-preventive COX-2 inhibitor agents (18). We have recently reported that two tri-aryl structure compounds (A, B) (Figure 1) displayed a significant anti-proliferative activity with considerable IC50 values (6.5 and 10.1 µM) on breast adenocarcinoma (MCF-7) cell line after 24 h of treatment (17). Considering these data and knowing that triple negative breast cancer is one of the most complicated subtypes of breast cancers with high aggressiveness and poor prognosis, we decided herein to evaluate the antitumor property of compounds A and B in both in-vitro and in-vivo models resembling the human triple negative breast cancer.
Experimental
Chemicals and cells
Human breast adenocarcinoma (MDA-MB-231, C578) and mouse mammary tumor (4T1, C604) cell lines were purchased from National Cell Bank of Pasteur Institute of Iran (NCBI). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco-BRL, Rockville, IN) containing 10% fetal bovine serum (FBS) (Gibco-BRL, Rockville, IN), and 1% Penicillin/Streptomycin (Gibco-BRL, Rockville, IN). Compounds A and B were synthesized in the medicinal chemistry laboratory at the faculty of pharmacy of Tehran University of Medical Sciences. Celecoxib was kindly provided by Pars Daru (Tehran, Iran). All other chemicals were in high purity and prepared from Merck (Darmstadt, Germany) and Sigma–Aldrich (StLouis, MO).
MTT assay
MTT assay was employed to assess the inhibitory effect of compounds A and B on the cell growth. To do it, MDA-MB-231 cells (5×103 cells/well) were seeded in 96-well plates and incubated at 37 °C in a humidified 5% CO2 incubator. Then, the cells were treated with various concentrations (0.1-100 µM) for 72 h. Untreated cells as well as 0.3% DMSO- treated cells served as negative and vehicle controls. Following addition 20 µL of (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT, 5 mg/mL), cells were further incubated at 37 °C for 4 h. The supernatants were then aspirated, and 200 µL of dimethylsulfoxide (DMSO) were used to dissolve purple formazan in each well. The plates were shaken for another 15 min and the absorbance was read using a Microplate Reader (Star Fax-2100, ST. Louis, USA) at 545 nm. The percentage of cytotoxicity was determined using the following formula.
% of cell cytotoxicity
100-[(Abs (drug)/ Abs (control)*100]
Animals
A total of 98 female 6-8 weeks-old BALB/c mice used in our study were prepared from the National Animal Center (Pasteur Institute of Karaj) and maintained in a 12/12-h light–dark cycle, with food and water supplied ad libitum. The animals were treated in accordance with the guidelines approved by the animal ethics committee of Pasteur Institute of Iran. After that, exponentially 4T1 cells were trypsinized and 106 cells were re-suspended in PBS and inoculated into the mammary fat pad of the mice. Simultaneously, compounds A and B at doses of 1, 5, 10, and 15 mg/kg/day were i.p. administered five times a week for four weeks. Animal weight and tumor volume were measured once per week. The tumor volumes (mm3) were calculated in two dimensions using a digital caliper through the following formula: (length×width2)/2.
Histopathology
For histopathological analysis, primary tumors and lungs, the major metastatic organ, were collected. Tumors and organs were removed at day 28 post-tumor injection and fixed in 10% buffered formalin for at least 24 h and then processed for routine paraffin embedding. Five-micron sections of each lesion were stained with hematoxylin–eosin (H&E).
Immunohistochemistry
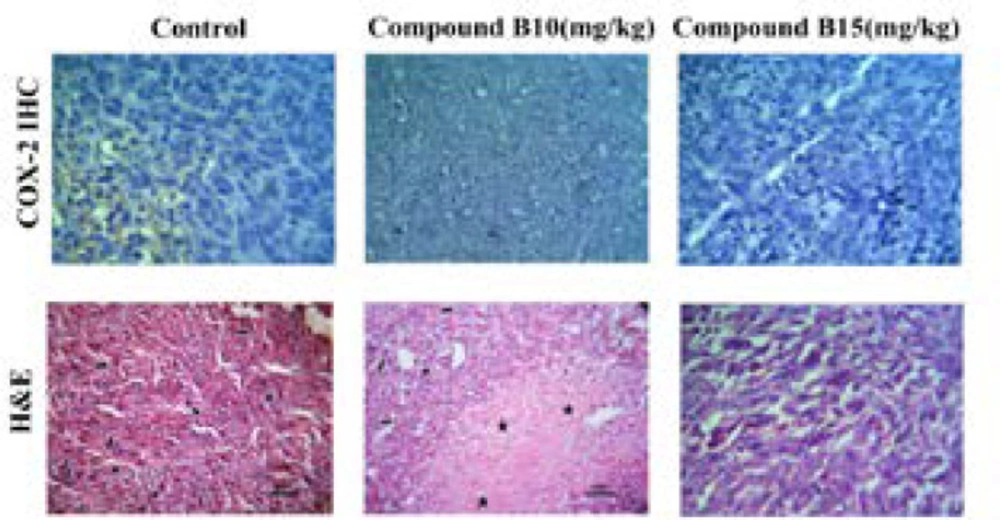
Immuno-histochemical assay was carried out on representative blocks of tumor tissues dissected from the mice treated with 10 and 15 mg/kg of compound B. Paraffin-embedded tumor sections (4 µm) were deparaffinized in xylene and rehydrated by graded alcohol and then incubated with anti- COX-2 antibody (RCM 306A, Biocare) followed by biotinylated secondary antibody using an HRP/DAB detection IHC kit (ab64264, Abcam, Cambridge, MA 02139-1517 ,UK) according to the manufacturer’s instructions and finally analyzed by an expert pathologist. Suitable positive control was run with each experiment.
Statistical analysis
The presented data are mean ± SEM of at least triplicate determinations, and the comparisons were based on ANOVA followed by the Tukey’s post test using GraphPad Prism software version 6. A p value lower than 0.05 was considered as significant.
Results
Anti-proliferative activity of compounds A and B
Anti-proliferative effects of compounds A and B were assessed by using MTT assay against invasive human breast cancer cell (MDA-MB-231) and the IC50 values were reported in (Table 1). The results revealed a potent anti-proliferative effect against cancer cells for compound B after 72 h of incubation with the IC50 value of 9.2 µM. Considering the IC50 values in (Table 1), compound B was superior to compound A in inhibiting the invasive and proliferative cells (9.2 vs. 17.95 µM ). The highest anti-proliferative activity was for celecoxib displaying an IC50 value of 7.45 µM.
In-vivo inhibition efficiency
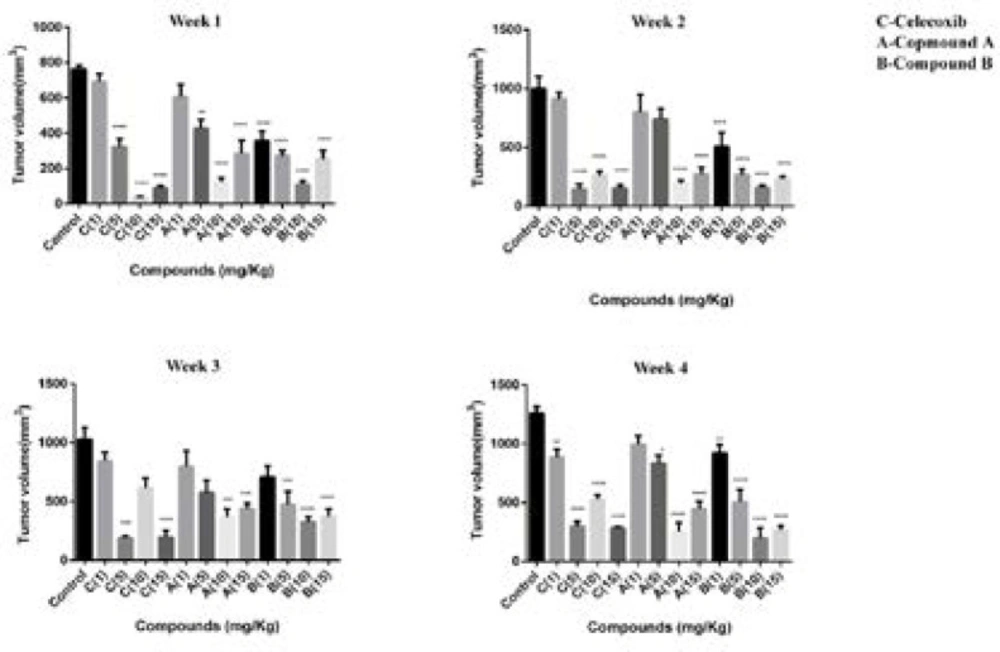
In our previous study, we reported firstly that two compounds (A and B) with tri-aryl structures displayed anti-breast cancer activity by the mechanism of cell apoptosis induction through a COX-2-independent pathway (17). The current work further confirmed our previous results in an in-vivo model. Hence, a 4T1 mammary carcinoma model was used to explore the potential properties of compounds A and B on breast cancer growth and metastasis. In this model, 4T1 cells were inoculated into fat mammary pad of BALB/c mice. Then, the mice were divided into 14 groups and treated with celecoxib, compounds A, and B. The i.p. administration was performed at an interval of 7 days for 5 times at doses of 1, 5, 10, and 15 mg/kg/day for 4 weeks. As depicted in Figure 2, administration of compounds A and B as well as celecoxib at doses of 1,5,10,15 mg/kg/day resulted in primary tumor size regression within the four weeks treatment; however, tumor size reduction was the most significant at week 4.
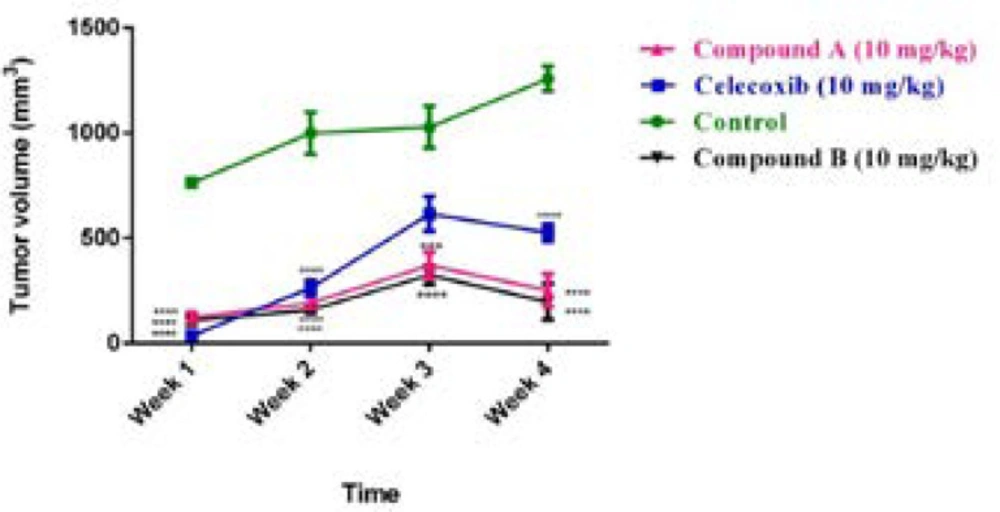
Our results also demonstrated that after treating for 28 days, the average tumor volume of the control group was about 1250 mm3. However, tumor size in mice was significantly reduced with treatment of both compounds and celecoxib. The mice treated with compound A had the average tumor volumes about 990, 830, 250, and 440 mm3 at doses of 1,5,10,15 mg/kg/day, respectively, after 4 weeks of administration. The compound-B treated mice displayed better inhibition effect on tumor volume than compound A. Our results indicated that tumor sizes in the mice treated with 1, 5, 10, 15 mg/kg/day of compound B were 920, 505, 197, and 268 mm3 at week 4. For celecoxib, tumor volumes were as follows at the mentioned doses after 4 weeks of treatment; 887, 300, 526, and 284 mm3 (Figure 3).
Our findings demonstrated that lower doses of compounds A and B (1 and 5 mg/kg/day) were unable to remarkably block the tumor progression; however, the animals treated with 10 mg/kg/day of both compounds displayed a more notable tumor size reduction compared with those treated with 1 and 5 mg/kg/day suggesting a dose-dependent anti-mammary tumor effect of the compounds. Interestingly, both compounds followed the same trend in the 4 -week treatment period and the most effective response was seen at the end of the study after 20 days of administration. In addition, celecoxib diminished the tumor size at different concentrations; however, among the various concentrations administered, 5 mg/kg/day of celecoxib was enough effective to reduce the tumor volume within the last three weeks of treatment, which may be attributed to the high potency of celecoxib compared with the two compounds. Interestingly, with the increasing dose of celecoxib to 10 mg/kg/day, its potential to repress the tumor growth was reduced. These findings suggest a negative feedback mechanism for celecoxib, which is line with a study performed by Ramer et al.(19). Noteworthy is mentioning that 10 mg/kg of daily administration of celecoxib was more effective than 5 mg/kg at the first week of treatment in tumor reduction size implying a time-dependent uptake efficiency for celecoxib. Based on our results obtained herein, we selected the dose of 10 mg/kg/day of the compounds to be administered by which the tumor size reduction was utmost and we subsequently carried out our further experiments at this dose. Surprisingly, with increasing the administered dose to 15 mg/kg/day, no effective response by tumor size reduction was observed in the mice treated with compounds A and B.
Moreover, no obvious indication of morphological change as well as weight loss was observed in the mice treated with compounds.
Compounds A and B enhanced tumor necrosis
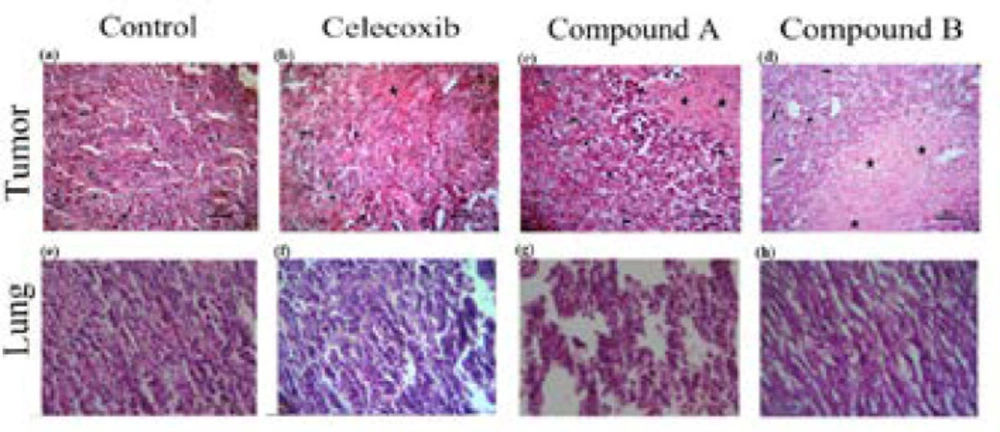
To confirm our in- vivo data and show whether compounds A and B demonstrate anti-tumor activity, histopathology studies were performed on tumor sections upon sacrificing mice on day 28. Based on the H&E staining results, as illustrated in (Figure 4) (a-d), the control group was composed of the enormous neoplastic epithelial cells possessing large round to oval nuclei and small to moderate amounts of eosinophilic cytoplasm. Also, a number of mitotic cells were remarkably observed in the tumor sections of control group. However, the sections of the tumor masses from the treatment groups, either compounds A and B or celecoxib, displayed differences in degree of necrosis; i.e. the lesion from the compound B-treated group revealed a marked necrosis within the tumor mass with less inflammatory cells at the periphery of the lesion than that of control and compound A treated-group at the same dose administered (10 mg/kg/day). Interestingly, the observed necrosis was even more than that of celecoxib-treated group
Effect of the compounds on lung metastasis
A hallmark of the malignant tumors is metastasis (21, 22). Thus, herein, we evaluated the anti-metastatic effect of the two compounds in the 4T1 tumor-bearing mouse model, which closely mimics metastatic breast cancer in human (23). 4T1 cells are approved to be highly invasive and primary cells usually metastasize to the lung following establishment for 2 to 3 weeks in BALB/c mice (23). In the current study, we investigated the effect of compounds A and B on the metastasis of breast cancer using 4T1 mammary carcinoma cells, which indicates a highly tumorigenic and invasive feature. To do it, the mice were killed and the lungs were subsequently dissected on the 28th day following 4T1 cells inoculated (Figure 4 e-h). As shown in (Figure 4,e-h), compounds A and B suppressed the metastatic potential of 4T1 tumor cells. Metastasis incidence was seen in vehicle group, while the presence of metastatic cells in the pulmonary of compound-B treated group was significantly less than that of the compound A-treated group at the same dose (10 mg/kg/dose). 10 mg/kg/day of celecoxib treatment did not drastically diminish the number and size of lung metastasis nodules. This is the first report demonstrating that both compounds are effective not only in controlling the primary tumor size, but also in suppressing secondary lung metastasis.
The association between expression of COX2 and tumor size
According to the results of our in-vivo experiment, compound B at dose of 10 mg/kg/day was more effective than that of 15 mg/kg/day, in terms of tumor reduction size. In order to verify whether this effect may be due to an increase in COX-2 expression, IHC was applied to detect COX-2 immunopositive cells in the cytoplasm (Figure 5). The findings exhibited that COX-2 protein was weakly expressed in the tumors dissected from the control mice after 28 days. However, compound B at the both doses administered could inhibit COX-2 expression suggesting no association between COX-2 expression and tumor reduction size in compound B treated group at doses of 10 and 15 mg/kg/day.
These results supported our previous study and suggest a target other than COX-2 for the antitumor effect of compound B (17). Since VEGF is a prerequisite for tumor invasion and its expression may be through either COX-2 dependent or independent pathway (20), the further studies are still deserved to validate VEGF as a target responsible for the conflicting results at the higher dose.
Tumor volume (mm3) in BALB/c mice with mammary cancer (4T1 breast cancer model) treated by celecoxib, compounds A and B at doses of 1,5,10 and 15 mg/kg/day for 4 weeks. One-way ANOVA test (post-Tukey test) done for n = 7 mice per group. Error bar indicates SEM, (**p < 0.01, ***p < 0.001, ****p < 0.0001 compared to the control group).
Effect of celecoxib and compounds A and B at dose of 10 mg/kg on solid tumors and lungs in Balb/c mice injected with 4T1 cells. The mice were killed 28 days after cell injection, tumor (a-d) and lung (e-h) sections were evaluated by H&E staining (original magnification ×200). Small arrow: inflammatory cells; Big arrow: tumor cells; Star: necrotic area; Headache: mitotic division
Expression of COX-2 in tumor tissues. Tumor tissues were collected from the mice receiving 10 and 15 mg/kg /day of compound B. Representative images of the immunohistochemical analysis are shown. All photomicrographs are at ×200 magnification. Small arrow: inflammatory cells; Big arrow: tumor cells; Star: necrotic area; Headache: mitotic division
| Compounds | IC50 (µM) |
|---|---|
| Celecoxib | 7.45 (6.56-8.45) |
IC50 values (µM) for cytotoxic activity of compounds A and B towards MDA-MB-231 Cells.a
Conclusion
In conclusion, our findings presented the first evidence of the ability of compounds A and B with tri-aryl structures resembling COX-2 inhibitors to prevent the tumor growth as well as metastasis to the lung against breast cancer. Our findings also suggest an underlying mechanism of the higher dose of compound B at which it was unable to inhibit the tumor growth. This occurrence may be attributed to the elevated level of VEGF expression through a COX-2 independent pathway. Overall, our data propose the use of compound B as a co-therapy in the protocols of cancer treatment; however, it needs further experiments to be validated.