Introduction
In December 2019, the city of Wuhan in Hubei Province, China, became the center of an outbreak of pneumonia of unknown cause. On January 7, 2020, the Chinese health officials confirmed the identification of a novel coronavirus (COVID-19). COVID-19 from Wuhan in China is currently causing concern in the medical community as the virus is spreading around the world. So far, 102,242 cases of COVID-19 have been clinically confirmed and 3,497 deaths have occurred till March 7, 2020. COVID-19 has a significant burden worldwide as a pandemic disease. The clinical presentation of COVID-19 pneumonia may present from mild to severe illness including Acute Respiratory Distress Syndrome (ARDS). Remarkably, the critically ill patients with COVID-19 are more likely to develop ARDS regarding the cytokine cascade activation over a short period of time (1, 2). The most common complication is ARDS, which is seen in the COVID-19 patients with high mortality rates.
Experimental
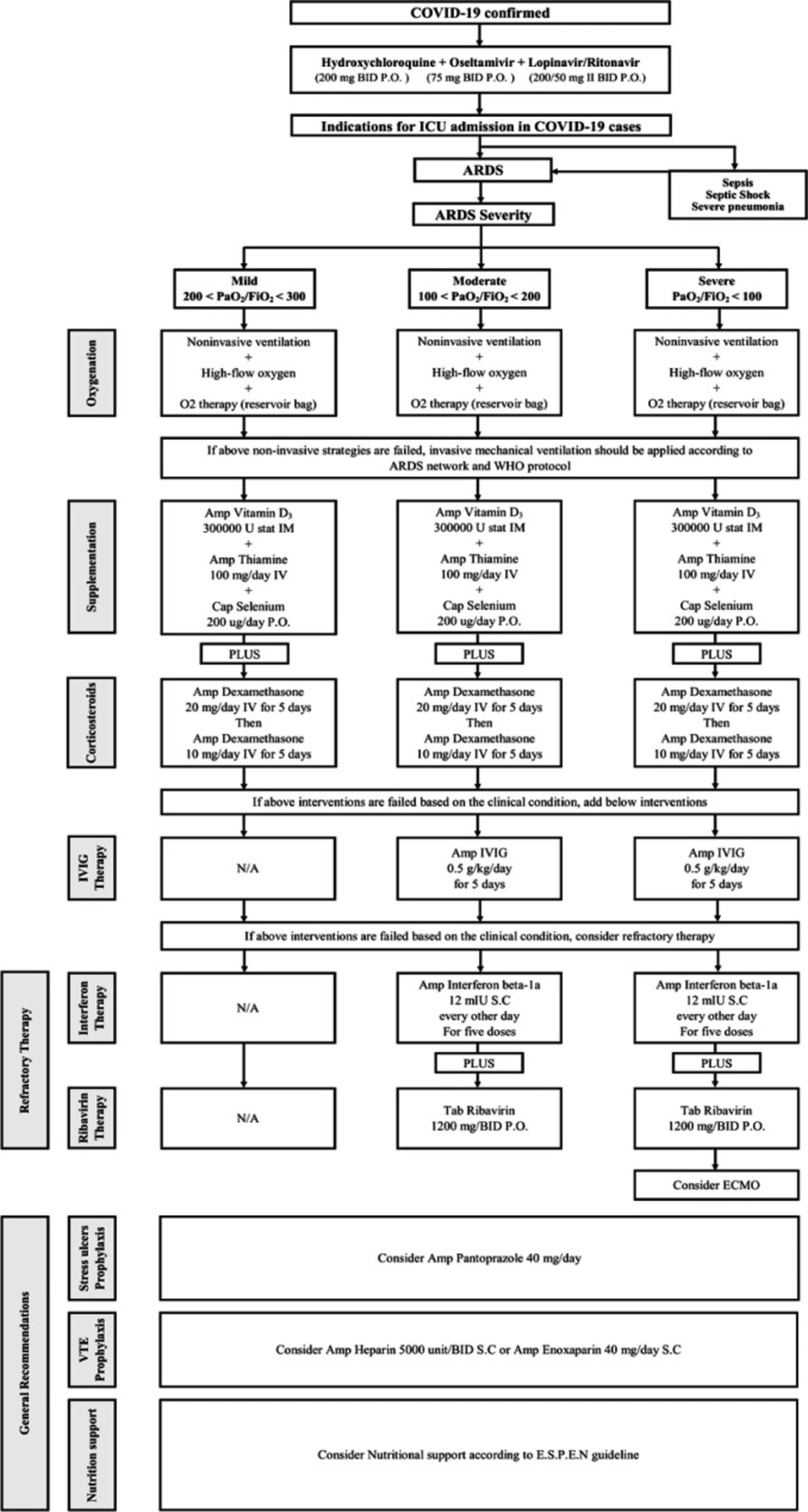
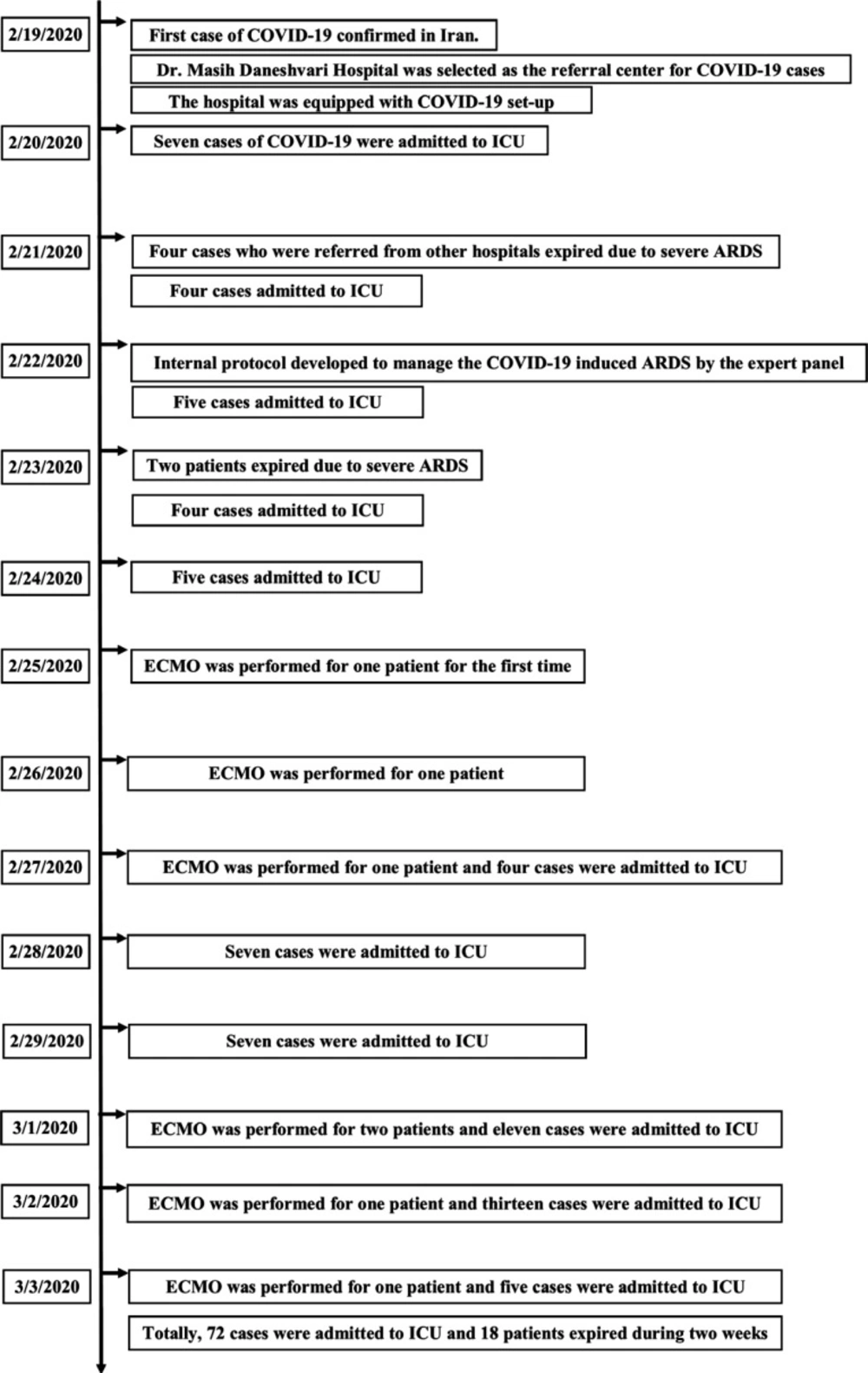
On February 19, 2020, Iran reported its first confirmed cases of COVID-19 and Dr. Masih Daneshvari Hospital was selected as a referral center for COVID-19 cases. The hospital was equipped with a special set-up to admit COVID-19 cases. On February 20, 2020, seven patients were admitted to the hospital with the confirmed COVID-19. In the first 30 h, four cases COVID-19 who were referred from the other hospitals and admitted in the intensive care unit (ICU) died due to severe ARDS. Because of the rapid progression of ARDS, an expert panel was held on 22 February 2020, consisting of all intensivists, infectious disease specialists, pulmonologists, internal medicine specialist, cardiology specialist, and clinical pharmacy specialist. Finally, an internal protocol was developed to manage the COVID-19 induced ARDS according to WHO recommendations and NIH guidelines (3). Different therapeutic regimens were employed on this protocol based on the ARDS severity and the patients’ special characteristics. This protocol is shown in Figure 1.
Of the 231 suspected cases of COVID-19 admitted to the hospital during two weeks, 72 patients were admitted to ICU with diagnosis confirmed by RT-PCR. In total, mortality in the ICU was 25% (n = 18) among ARDS patients over two weeks (Table 1).
Twenty-four patients recovered and were transferred to the ward. Thirty patients were under treatment in ICU. Totally, 29 patients expired during two weeks including 18 cases in ICU. The timeline of two-week events is depicted in Figure 2.
| Non-Survivor (%) | Survivor (%) | Number (%) | Group |
|---|---|---|---|
| 3 (16.7) | 18 (33.3) | 21 (29.2) | Mild ARDS |
| 4 (22.2) | 31 (57.4) | 35 (48.6) | Moderate ARDS |
| 11 (61.1) | 5 (9.3) | 16 (22.2) | Severe ARDS |
| 18 (100) | 54 (100) | 72 (100) | Total |
Fourteen days mortality at a glance
Results and Discussion
Protocol Recommendations
The patients’ vital signs, CBC, serum creatinine, urea, urine output, CRP, LFT, bilirubin, coagulation parameters, ABG, and regular chest imaging must be closely monitored.
All of the patients were administered hydroxychloroquine, oseltamivir, and lopinavir-ritonavir before admission to ICU based on the Ministry of Health protocol and recent data regarding COVID-19 treatment (4, 5).
Oxygenation
Oxygen therapy, non-invasive ventilation or invasive mechanical ventilation are considered based on O2 saturation according to the WHO recommendations and NIH guidelines. The patients were categorized into three groups based on PiO2/FiO2. According to Berlin criteria, mild, moderate, and severe ADRS are defined as PiO2/FiO2 between 200 and 300, 100 and 200, and less than 100, respectively. Invasive mechanical ventilation should be applied to ARDS patients with persistent hypoxemia.
Supplementation
We recommend the administration of vitamin D3, thiamine, and selenium in the ARDS patients according to our protocol. Vitamin D deficiency is associated with greater cellular inflammation and cytokine release at 48 h after ARDS development. Thiamine deficiency is also a risk factor for ARDS development based on several published trials, whereas selenium has a beneficial role in oxidative stress pathways.
Steroid therapy
As we have faced with the rapid progression of ARDS in our cases, the administration of corticosteroid is recommended for all our patients to inhibit the inflammation process in ARDS. High doses of dexamethasone as 20 mg daily from day 1 to 5, then 10 mg daily from day 6 to 10 were administered in all stages of ARDS, even in the mild cases. This approach was applied based on the study done by Villar et al. and the rapid progression of ARDS in our cases in order not to lose the time (6).
Immunoglobulin Therapy
IVIG is administered in the cases that failed with the above strategies. IVIG has controversial effects on the patients with respiratory coronavirus. IVIG is applied to treat some diseases, especially primary immune deficiencies, autoimmune neuromuscular disorders, and respiratory failure regarding sepsis. It is considered as an effective agent in the cases of coronavirus associated fulminant myocarditis (7, 8).
Interferon beta-1a and Ribavirin Combination
Combination therapy with interferon Beta-1a and ribavirin in all refractory cases is administered based on data regarding coronavirus treatment and Ebola experiences.
Extracorporeal membrane oxygenation (ECMO) and Refractory Therapy
ECMO is a specialized, resource-intensive, and expensive form of life support. However, it is associated with severe complications including nosocomial infection and hemorrhage. ECMO can serve as a life-saving rescue therapy for refractory respiratory failure in the setting of ARDS, such as that induced by coronavirus disease 2019 (COVID-19) (9).
Other therapies
Stress ulcer prophylaxis, venous thromboembolism prophylaxis, and nutrition management are applied to all of the patients.