Introduction
Mycotoxins are a group of thermally stable low molecular weight (0.3–0.7 kDa) toxic compounds produced by a wide variety of fungal species as their secondary metabolites with a high bioaccumulation ability (1, 2). According to literature, aflatoxins (AFs) are noted as the most studied mycotoxins, among more than 300 identified fungal secondary compounds (3), including B1, B2, G1, and G2. AFs produced primarily by Aspergillus flavus (produces aflatoxins B1 and B2) and A. parasiticus (produces all B1, B2, G1 and G2) (4). Food contaminated by AFs may cause several health problems in consumers, including acute or chronic diseases with mutagenic, carcinogenic, and teratogenic effects (5); hence, AFB1, as the most acute hepatocarcinogenic aflatoxin, is classified in group 1: “carcinogenic to humans” by the International Agency for Research on Cancer (IARC) (6). Additionally, based on a report by Raiola A, Tenore GC, Manyes L, Meca G and Ritieni A (7), children exposed to AFs are associated with reduced immunization efficiency, consequently increased susceptibility to infections as well as stunted growth.
A variety of food products (agricultural commodities) can be contaminated with AFs due to either infected grains by fungi or post-harvest contamination (8). Cereal and cereal-based products contaminated with AFs are a global health concern as they are used worldwide to supplement foods in babies’ diets, providing them with energy and nutrients when breast milk is no longer enough to meet their nutritional needs (9). Daily consumption of AFs contaminated cereal-based baby food increases the associated risks to babies’ health, such as stunted growth and deficiencies in warding off infectious diseases. (10). Furthermore, incomplete enzymatic systems in children and babies to eliminate hazardous compounds (11, 12) and their higher sensitivity to toxicogenic compounds make them more vulnerable to AFs and leave them at risk of developing chronic diseases later in life (13). Accordingly, due to extreme health concerns regarding contaminated babies’ diet due to AFs, numerous national and international governmental authorities on public health have established maximum regulatory levels for AFB1 i.e., the maximum level established by European Union is 0.10 ng/g in Commission Regulation (EC) No 1881/2006 (14) and by National Standard Organization of Iran is 1.00 ng/g for food products without milk and 0.5 ng/g with milk in infant and baby foods (15).
As the elimination of AFs from food products is extremely difficult, babies are exposed to a high risk of consuming AFs contaminated foods. Therefore, continuous monitoring and dietary risk assessment of AFs in baby food products are necessary to provide critical information for food regulators in setting guideline values for AFs in food products. The risk assessment process is based on a series of steps, including hazard identification, hazard characterization; exposure evaluation; and risk characterization. This approach is one of the best practices when applied to comply with new regulations set by international organizations or developed regions (16). The results from a risk assessment can enable risk managers to effectively establish mitigation strategies or contingency plans for accepting any risks. In terms of evaluating exposure to aflatoxins, there are three different methods that can be considered, namely: point estimate (or deterministic) procedure; the semi-probabilistic method; and the probabilistic model, also known as the Monte Carlo simulation (MCS) (17). The latter method is the most promising due to its capability in identifying uncertainties and variabilities in the long-term exposure estimations as well as in performing risk predictions that are used extensively in food sciences (18). Therefore, in this study, firstly, the AFs occurrence in different cereal-based baby food samples from different commercial brands from Iran market in two cold and warm seasons were analyzed and then dietary exposure to AFs via cereal-based baby foods consumption and consequently risk characterization was estimated by MCS in three different age groups including babies aged 6-12, 12-18, and 18-24 months.
Experimental
Chemicals and standards
AFs standards (B1, B2, G1, G2), methanol, acetonitrile, ultrapure water, potassium dihydrogen phosphate, potassium chloride, anhydrous disodium hydrogen phosphate, sodium chloride, nitric acid and potassium bromide, all HPLC-grade with high purity, were purchased from Merck (Darmstadt, Germany). Standard solutions of AFB1 and AFG1 at 1000 ng/mL and AFB2 and AFG2 at 200 ng/mL concentrations were prepared weekly and used in order to obtain calibration curves.
Samples collection
One hundred and twenty cereal-based commercially available baby food samples from six different manufacturers were purchased from retail outlets in Iran market. According to their production date, samples were classified into two categories: cold season (winter) collected in February 2020 and warm season (summer) collected in August 2020 according to their production date. Forty samples from six brands were prepared so that each sample was a mixture of three separately purchased samples of the same brand collected from different batches during a month (19). The collected samples were kept at 4 ºC until further analysis. However, based on the products’ labels, all samples were suitable for infant consumption from six months after birth, and their main ingredients mostly included flour of wheat, rice, maize, oat and rye, although, in 6 of them, almond was the main ingredient. Most of the purchased samples were originally in powder form (n = 32) and the rest in semi-solid-to semi-liquid (n = 8).
AFs determination
AFs extractions
The extractions were carried out based on Iranian Standard No.6872 with a slight modification (20). Briefly, 20 g of each sample were homogenized and grounded using a blender (Waring, The USA) for 5 min at 18000 rpm. Then, the homogenized samples were well mixed with 100 mL of 80% methanol in water (v/v) in a conical flask for 5 min more at 18000 rpm in order to extract aflatoxins from the samples’ matrix and filtered through a no. 5 Whatman filter paper. Twenty milliliters of each filtrate were then diluted to a final volume of 100 mL using phosphate-buffered saline (PBS) (pH=7.4) in a microwave glass container and shook vigorously. A 110 mL of the obtained solution was filtered using a filter paper (Whatman no. 5) and slowly loaded in an immunoaffinity column (Libios, France) where aflatoxins can trap as antigen inside the column. The column was then washed with 2 mL acetonitrile and dried under air, afterward eluted with 0.5 mL of 60% methanol in water (v/v), and the extracted AFs were collected and kept at 4 °C until further analysis.
AFs analysis
The amounts of AFs were determined using HPL-FLD according to a method established by the Institute of Standard and Industrial Research of Iran (ISIRI; Iranian Standard No. 6872) with some modification (20). The HPLC system consisted of liquid chromatography (Waters e2695, separation module, USA), equipped with a Fluorescence detector (model 2475) that was set 365 nm as the excitation wavelength and 435 nm as the emission wavelength and a quaternary, low-pressure mixing pump and inline vacuum degassing. The sensitivity of the instrument was set on 500 EUFS. Chromatographic separation was performed in an Octadecyl-silica (ODS) column (150 ×4.6 mm I.D., 3 µm) (GL Science) with a column oven which kept the column temperature constant at 50 °C throughout the AFs separation. An isocratic elution mobile phase of acetonitrile, methanol, and HPLC-grade water (2:3:5, v/v) containing 35 μL nitric acid and 12 mg KBr (to derivatization in Kobra cell (Farlib, France)) was used with a flow rate of 1.8 mL/min for a sample volume of 75 µL and a separation time of 10 min. The levels of AFs were determined by comparison of retention times and area values with corresponding standards, and the results were expressed as part per billion (ppb).
Method validation
The performance of the used method in the current study for the determination of AFs was evaluated in terms of linearity, the limit of detection (LOD), the limit of quantification (LOQ), recovery, and precision. LODs and LOQs were determined based on a signal-to-noise ratio (S/N) of 3 and 10, respectively, where samples were spiked with different concentrations of AFs. The linearity was determined by injecting different concentrations of AFB1 and AFG1 at 0.02, 0.06, 0.1, 0.14, 0.18, 0.28, 0.36, 0.64, 0.88, and 1 ng/mL and also AFB2 and AFG2 at 0.004, 0.012, 0.020, 0.028, 0.036, 0.056, 0.072, 0.110, 0.150, and 0.200 ng/mL in duplicate. The percent of recovery also was calculated by comparing 0.5 ng/mL of mixed AFs standards to baby food samples with the measured concentrations after three times analysis. Within-day (run-to-run) precision of the HPLC-FLD method, expressed as RSDs%, was calculated by extracting and analyzing AFs in one sample seven times at the same condition (21).
Dietary exposure estimation
Dietary exposure to AFs categorized into two groups (AFB1 and total AFs(sum of AFB1, AFB2, AFG1, and AFG2)) were estimated for three different age groups, including babies aged 6-12 months, 12-18 months, and 18-24 months in the Iran population using the following formula (22):
Equation 1.
where EDI is estimated dietary intake (ng/kg BW/day), C is the amount of different AFs levels, and F is the consumption rate of cereal-based baby food assumed to be 100 g/day based on recommended serving size for each brand by producer and using scientific literature reports (23). It should be noted that the body weight ranges for each considered group (6-12 months, 12-18 months, and 18-24 months) assumed to be 7.5-9.6, 9.2-10.9 and 10.6-11.9, respectively, according to WHO standards (24). The C, F, and Body weight followed log-normal, triangle and uniform distributions, respectively, which were used then for probabilistic analysis (Monte Carlo simulation approach) of the estimation of dietary exposure using Crystal ball software (version 11.1.2.3 Oracle) (17). For each age group and both AFB1 and total AFs, the Monte Carlo simulation was repeated 100,000 times in order to achieve a dietary intake as accurately as possible and also forecasted 5, 50 and 95 percentiles of the consumer population.
Health Risk Assessment
The Margin of Exposure (MoE)
In general, benchmark dose lower confidence limit 10% (BMDL10), which is an estimation of the lowest dose that is 95% certain to cause no more than 10% cancer incidence, is suggested to obtain MoE. However, the MoE was estimated by dividing the BMDL10 reference value of 400 ng/kg BW/day (25) to 5, 50, and 95 percentiles exposures of AFB1 and total AFs, using the Monte Carlo simulation approach. The MoE values, based on the BMDL10 from an animal study, equal or higher than 10,000 are considered to be a low public health concern (26).
Equation 2.
Cancer Risk
According to an assessment by EFSA (CONTAM Panel), the cancer potency for both AFB1 and total aflatoxins is counted to be equal. It reported exposure to aflatoxins to induce 0.01 and 0.3 additional cancer cases per 100,000 for HBsAg− and HBsAg+ populations, respectively (25). Taking this into consideration and according to the prevalence of HBsAg+ in Iran (1.7%) (27), the cancer risk due to AFB1 and total AFs exposures-induced hepatocellular carcinoma was obtained for 5, 50, and 95 percentiles. Therefore, to determine cancer probability, the percentage of the population for both carrier (Pop.HBsAg+% = 0.017) and non-carriers (Pop.HBsAg-% = 0.983) of hepatitis B virus infection in Iran and the carcinogenic potency reported by EFSA (PHBsAg- and PHBsAg+ = 0.01 and 0.3 respectively) put into following formula:
Pcancer = (PHBsAg+ × PopHBsAg +%) + (PHBsAg- × PopHBsAg -%)
Statistical analysis
SPSS software v. 26 and Crystal ball software (version 11.1.2.3 Oracle) were applied in this study. As one sample K-S test showed the obtained data were not normal; therefore, a nonparametric test was used (Mann-Whitney) to compare samples from different seasons in terms of aflatoxins contamination (p < 0.05). Moreover, Crystal ball software was used to risk assessment values calculations.
Results and Discussion
Method performance and Validation
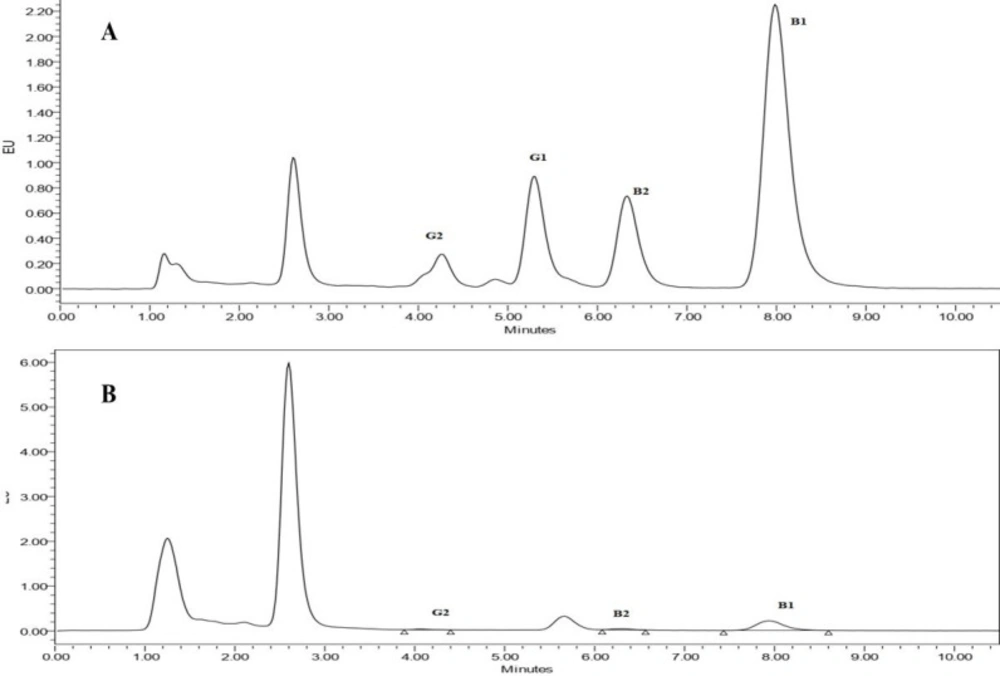
In risk assessment studies, reliable analytical methods for risk measurements are required since these data are used to calculate risk factors, which allow risk managers to effectively establish mitigation strategies or contingency plans for accepting any risks. Accordingly, it is internationally authenticated that an appropriate method must be used to ensure precise results. Thus, method validation has received great attention from industrial committees and regulatory agencies (28). For that reason, the performance of the HPLC-FLD method, the used method in the current study, was evaluated based on recovery percentage, the limit of detection (LOD), the limit of quantification (LOQ), coefficient of determination (R2), repeatability (RSD), and linear range. The levels of assessed AFB1 and AFG1 in the range of 0.02-1 ng/mL and the levels of AFB2 and AFG2 in the range of 0.004-0.2 ng/mL were linear with an R2 greater than 0.99 for all AFs. The RSDs for B1, B2, G1, and G2 aflatoxins were 7.2%, 7.1%, 6.3%, and 6.3%, respectively, calculated based on comparative peak areas of seven replicates of each sample, indicating good repeatability of the method used. The method also showed a high percentage of analytes recovery for B1, B2, and G2 aflatoxins with percentages of 92%, 105%, and 99%, respectively, while the recovery percentage for G1 was 70%. Considering a chromatographic signal to noise ratio of 3 for LOD and 10 for LOQ, the LODs of the method for B1, B2, G1, and G2 aflatoxins were 0.013 ng/g, 0.003 ng/g, 0.016 ng/g, 0.003 ng/g, respectively, and LOQs for B1, B2, G1, and G2 aflatoxins was 0.04 ng/g, 0.01 ng/g, 0.05 ng/g, 0.01 ng/g, respectively. These results taken together, confirm the validity and performance of the method used, which is compliant with European Union guidelines, as repeatability in terms of RSD% was lower than 20% and the recovery range was between 70–120% (SANTE/11813/2017. Guidance) (29). Comparing the obtained results to the performance criteria set out in the Commission Regulation (EC) No 401/2006 indicates that the method satisfies these performance criteria in terms of repeatability, recovery, and reproducibility for aflatoxins in baby food (30). Figure 1 illustrates the chromatograms obtained by HPLC-FLD for spiked (A) and non-spiked (B) cereal-based baby food samples. The concentration of aflatoxins in the spiked sample was 0.5 ng/mL for AFB1 and AFG1 and 0.1 ng/mL for AFB2 and AFG2.
AFs occurrence in analyzed samples
Forty different samples of cereal-based baby foods from available commercial brands in Iran market in two cold (20 samples) and warm (20 samples) seasons were analyzed for AFs (B1, B2, G1, and G2) contamination (Table 1). As Table 1 shows, the most frequent occurrence in the cold season was observed for AFB2, which was detected in 60% of assessed samples, followed by AFB1 (50%), while AFG1 and AFG2 were detected only in one (5%) and 4 samples (20%), respectively. On the other hand, the samples from the warm season showed the aflatoxins occurrence for both AFB1 and AFB2 were 60% (detected in 12 samples) and for AFG2 was 30% (detected in 6 samples); while AFG1 was not detected in any of the assessed samples. Only two samples taken from the cold season (0.51 ng/g and 0.84 ng/g) exceeded the maximum limit of AFB1 (0.5 ng/g) established by the National Standard Organization (15) of Iran; however, six of them (30%) had a concentration beyond European Commission (EU) maximum level of 0.1 ng/g for AFB1 (14), with contamination levels of 0.11, 0.14, 0.28, 0.5, 0.51, and 0.84 ng/g. Similarly, from the warm season samples, only one sample exceeded the AFB1 level established by the National Standard Organization of Iran, while seven; samples exceeded the European Commission maximum level for AFB1.
There are several studies associated with the presence of aflatoxins in cereals and cereal-based products; for example, Zhang K, Flannery BM, Oles CJ and Adeuya A (31) analyzed a total of 215 retail samples of commercial infant/toddler foods (cereals and teething biscuits) and breakfast cereals collected from three geographical locations in USA for aflatoxins contamination but did not detect any amounts of aflatoxins including B1, B2, G1 and G2 in any of the 215 analyzed samples. In another work, Ul Hassan Z, Al Thani R, A. Atia F, Al Meer S, Migheli Q and Jaoua S (32) reported the presence of AFB1 in 22% of 67 commercial formula milk and cereal-based baby food samples on the Qatar market which 14-43% of these positive samples had levels higher than the EU maximum limits, while none of B2, G1, and G2 were found at detectable levels in any of the assessed samples. In a similar study to our work, 18 samples out of 26 (69%) breakfast cereal samples purchased from supermarkets in Lisbon (Portugal) were contaminated with AFB1, 7 samples (27%) contaminated with AFB2 and one sample was contaminated with AFG1, while AFG2 was not detected in any of the analysed samples (33). The levels of AFB1, as the most carcinogenic aflatoxin, in the current study were also comparable to those reported by Ibáñez-Vea M, Martínez R, González-Peñas E, Lizarraga E and de Cerain AL (34) in breakfast cereal samples from the Spanish market (0.051–0.130 µg/kg), Villa P and Markaki P (35) (0.05–4.3 µg/kg of AFB1, with an incidence of 56.3%) in samples from Greece and Iqbal SZ, Rabbani T, Asi MR and Jinap S (36) (0.04–6.9 µg/kg of AFB1, with an incidence of 41%) in samples from the Pakistan markets. Both similarities and differences between the results of our study and the mentioned studies above could be due to a number of factors, including the differences in analytical methods, the source of cereal ingredients, geographical regions, climatic factors, seasonal variability, and product brands sampled. It is important to note that statistical comparison between AF levels in cold season and warm season were not remarkable (p > 0.05). The results were similar to a research caried out by Elaridi et al. who studied concentration of aflatoxin M1 and ochratoxin in cereal-based baby food samples in Lebanon. Similarly, the sampling was done in two different seasons and reported no significant difference between samples from different seasons (37).
Estimation of food consumption
In this study, the mean consumption rate of cereal-based baby food in babies aged up to 24 months (in three groups including 6-12 months, 12-18 months, and 18-24 months) was assumed to be 100 g/day based on recommended serving size for each brand by producers and using scientific literature reports about the average amounts of cereal-based baby food daily consumption (23). This amount was then used to calculate the estimated dietary exposure and subsequently estimate the risk assessment of consumption of cereal-based baby foods in studied age groups in this current work. In order to determine the mean consumption rate, some information was taken from a survey by the National Nutrition & Food Technology Research Institute of Iran (NNFTRI) (38); accordingly: 75.92% of babies in Iran start complementary food by solid, semi-solid, and hard complementary foods from ages ranged 6-8 months; 93.2% babies < 24 months were fed by complementary foods which 88.8% of them had the minimum meal frequency, and 84.4% of them had an acceptable variety in their daily complementary food. According the used questionnaire in NNFTRI survey 77.8% of babies consumed cereal-based complementary food the day before fill out the questionnaire, indicating the domination of the cereal-based foods as complementary food in the ages lower than 24 months in Iran.
Estimation of AFB1 and total AFs exposures
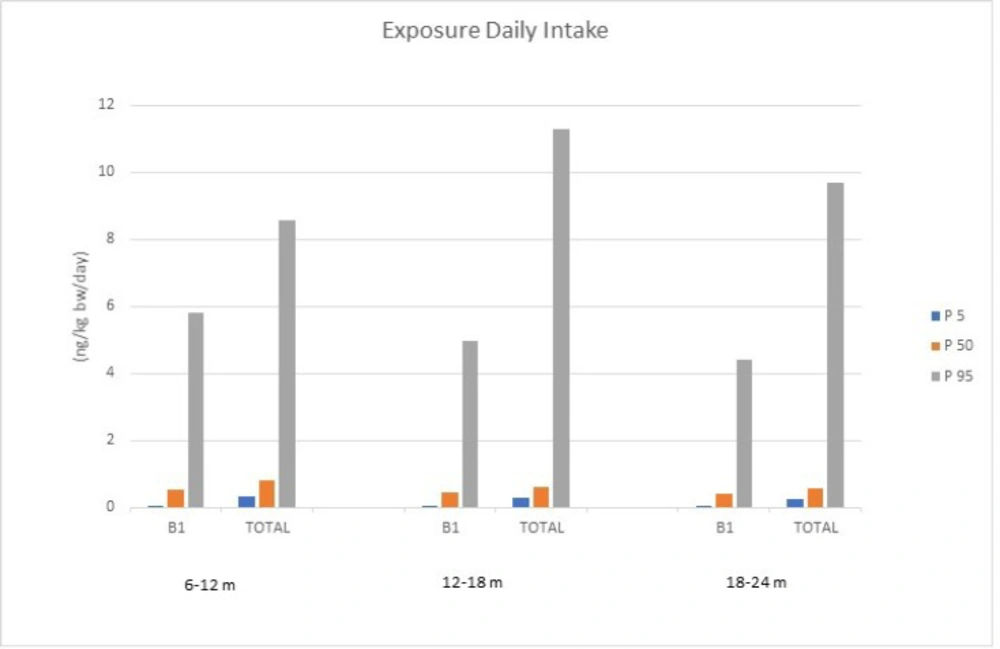
In general, the deterministic (point estimate procedure), semi-probabilistic method, and probabilistic modelling (Monte Carlo simulation) have been used to evaluate dietary exposure to hazardous compounds in foodstuff. The Monte Carlo simulation is a computational system for stochastic modelling, which has been considered the most promising method with the capability to make risk predictions and delete uncertainties (17). Consequently, the Monte Carlo simulation approach was used in order to estimate dietary exposure of three different age groups, including 6-12 months, 12-18 months, and 18-24 months to AFB1 and total AFs (TAFs; the sum of B1, B2, G1, and G2 aflatoxins). As is presented in Table 2, the calculations were performed for 5, 50, and, 95 percentiles and the simulation approach forecasted results as distribution. The Monte Carlo simulation model showed significant differences among different age groups with the highest estimated dietary exposure to both AFB1 and TAFs for 6-12 months aged babies, representing 5.81 ng/kg BW/day and 8.55 ng/kg BW/day regardless of the percentile, respectively. This can be explained by the lower body weight of babies in this group, as dietary exposure was obtained by multiplying the aflatoxins concentrations in the amount of consumption divided by body weight. Hence, lower body weight reflects a higher dietary exposure. Considering the results of the estimated dietary exposure using the Monte Carlo simulation approach for percentile 50 as the median of population, the lowest dietary exposure was recorded in 18-24 months age group for AFB1, and the highest dietary exposure for 6-12 months for TAFs, representing 0.41 ng/kg BW/day and 0.82 ng/kg BW/day, respectively. As is shown in Table 2, estimated dietary exposure results indicate a remarkable difference among the same age group for different percentiles; for instance, in the 6-12 months age group, 95% of the population (P5) had an estimated dietary exposure lower than 0.05 ng/kg BW/day to AFB1 indicating relatively low-risk exposure for this population, while 5% of this group (P95) was exposed to 5.81 ng/kg BW/day to TAFs, which is nearly 10-fold of estimated dietary exposure for the median of the studied population (P50). In the 6-12 months age group, for example, estimated dietary exposure to TAFs for percentile 5 (P5) was lower than 0.32 ng/kg BW/day, which is about one-third of the estimated dietary exposure for the median of population, while for 95 percentile this amount was quite high (8.55 ng/kg BW/day). For the other two age groups, 12-18 months and 18-24 months, 5% of the population (P95) estimated dietary exposure was relatively high compared to the median of the studied population (P50); this amount for the 12-18 months age group was 4.96 for AFB1 and 11.30 ng/kg BW/day for TAFs; and for 18-24 months the aged group was 4.39 for AFB1 and 9.69 ng/kg BW/day for TAFs, respectively; representing approximately a 10-fold of the median of these populations in the case of AFB1. Figure 2 depicts exposures to AFB1 and total AF in different groups and percentiles. However, as AFB1 is classified as the Group 1 of carcinogens to humans by the IARC, any threshold of dietary exposure for this toxin has not been established (6).
On the other hand, as aflatoxins may be widespread in many foodstuffs, achievement of “zero” dietary exposure seems to be impossible; therefore, it is desirable to reduce dietary exposure to aflatoxins to as low as possibly achievable (39). As the IARC (2015) has reported that long-term dietary exposure to aflatoxins leads to several health problems such as impaired or stunted growth in children, hepatocellular carcinoma (liver cancer), and long-lasting health complaints later in life (40). Therefore, it is vital that an assessment of dietary exposure to AFs in foodstuffs is conducted to show the current situation and determine a mitigation strategy to inform legislation policy to regulate the levels of aflatoxins in food. In this context, several countries have conducted research and have reported dietary exposure to aflatoxins through the consumption of cereal-based baby foods, which can be affected by either consuming a lot of moderately contaminated foods or eating a moderate amount of highly contaminated foods. In accordance with our results, Herrera M, Bervis N, Carramiñana JJ, Juan T, Herrera A, Ariño A and Lorán S (39) reported an estimated daily intake of AFB1 ranging from 0.17 to 0.37 ng/kg BW/day in cereal-based baby foods collected from a random sample of supermarkets, pharmacies and organic food retailers in the Cantabria and Aragón regions of Spain. Similarly, Bakker G, Sizoo E, Jekel A, Pereboom-De Fauw D, Schothorst R and Van Egmond H (41) reported an estimated daily intake of 0.42 ng AFB1/kg BW/day for children between 2–6 years old in the Netherlands. Cano-Sancho G, Sanchis V, Marín S and Ramos A (42) also stated that breakfast cereals are the main contributor to the total aflatoxin dietary intake for children with an estimated daily intake of 0.106 ± 0.113 ng/kg BW/day.
In another study, Ojuri OT, Ezekiel CN, Eskola MK, Šarkanj B, Babalola AD, Sulyok M, Hajšlová J, Elliott CT and Krska R (43) reported a dietary exposure of 5.5-51192 ng AFB1/kg BW/day (Median 528) from Tom bran (which is usually formulated from several whole grains including maize, peanuts, wheat, soybean and millet), 5.7-3211 ng AFB1/kg BW/day (median 20) from Ogi (a maize-based fermented gruel), 3.5-426 ng AFB1/kg BW/day (median 7) from infant formula (included products with a mix of milk and cereal (e.g., maize, oats, rice or wheat depending on the brand(, and 2.5 -639 ng AFB1/kg BW/day (median 91) from family cereal (a maize product) for infants and young children in Nigeria. They also reported a dietary exposure of 40.5-54892 ng/kg BW/day (median 641), 41.8-3539 ng/kg BW/day (median 68), 25.7-533 ng/kg BW/day (median 55), and 27-902 ng/kg BW/day (median 179) to TAFs through Tom bran, Ogi, infant formula, and family cereal diet, respectively. Similar to our results, the exposures varied through age groups; i.e., the mean exposure to AFB1 for 12–24 months age group (2985 ng/kg BW/day) was significantly higher than those for <12 months age group (282 ng/kg BW/day) as well as to the sum of aflatoxins the dietary exposure for 12–24 months age group and for under 12 months age group were 3840 ng/kg BW/day and 387 ng/kg BW/day, respectively. This observation demonstrates the correlation between the age of the child and the exposure increase, in that as a child grows up, its diet changes from mother’s breast milk to baby formula and breakfast cereals which are highly prone to mycotoxin contamination (44).
Risk characterization
The margin of Exposure estimation
Due to geno-toxicity and carcinogenicity of aflatoxins, the Joint FAO/WHO Expert Committee on Food Additives (JECFA) has not set a tolerable daily intake for them; hence, the risk characterization for aflatoxins is based on margins of exposure (MoE) (45, 46). Indeed, MoE is a ratio between the toxicity effects and dose of a hazardous compound which causes a low but measurable response (benchmark dose, BMD) and the estimated dietary exposure (23). In general, the benchmark dose lower confidence limit of 10% (BMDL10) has been suggested in order to calculate MoE, especially in the case of aflatoxins, which is an estimation of the lowest dose that is 95% certain to cause no more than 10% cancer incidence (47). According to the EFSA scientific committee guidance, MoE values equal to or higher than 10,000, based on the BMDL10 from an animal study, are considered a cut-off point of low concern from a public health point of view and can be assumed as a low priority for risk management actions (25). However, the output results from the Monte Carlo simulation method showed, except about percentile 95 for AFB1 in 18-24 months age group, an estimated MoE lower than 10,000 for the all percentiles (5, 50, and 95 percentiles) for all age groups calculated in this study to both AFB1 and TAFs, indicating a health risk from AFB1 and TAFs exposure through cereal-based baby food consumption in less than 24 months age population. Table 3 shows the forecasted distribution for MoE of aflatoxin B1 and total aflatoxin obtained from the Monte Carlo simulation. The 5th percentile, as the worst scenario (those who are at high exposure), represented a MoE of 695, 915, and 905 for AFB1 in 6-12, 12-18-, and 18-24-months age groups, respectively, and zero for TAFs in all age groups. These results are comparable with those reported by Assunção R, Martins C, Vasco E, Jager A, Oliveira C, Cunha SC, Fernandes JO, Nunes B, Loureiro S and Alvito P (48), who obtained a MoE below 10,000 of AFB1 through consumption of breakfast cereals, infant cereals, and biscuits by Portuguese children. A potential health risk increase due to the consumption of breakfast cereals containing aflatoxins, especially in those with a high consuming amount (percentiles 90, 95, and 99) has also been stated by Assuncao R, Vasco E, Nunes B, Loureiro S, Martins C and Alvito P (49); the AFB1 was the major contributor for the risk with a total MoE below 10,000. Based on the results of this study, it may be postulated that consumption cereal-based baby food from Iran market could lead to increased health risk in children aged < 24 months, which the higher risk in this age group can be explained by an exceptionally high intake in infants and children in relation to their body weight (50).
Cancer risk
According to cancer potency estimation and the prevalence of HBsAg+ in Iran (1.7%) (51) , the results indicate a high cancer risk for high consumers (worst scenario, P95) of cereal-based baby food in all age groups i.e. an estimation of 0.08, 0.07, and 0.06 additional cancer cases per 100,000 in the case of AFB1 exposure and 0.12, 0.16, and 0.14 additional cancer cases per 100,000 in the case TAFs exposure in the 6-12 months, 12-18 months, and 18-24 months age groups, respectively (Table 3), indeed, aflatoxins exposure through cereal-based baby food consumption can roughly add 1.5 new liver cancer patient per 1 million in a year. Given that AFs particularly AFB1 are highly carcinogenic and cause hepatocellular carcinoma in humans and it is important to consider immediate intervention (6, 44).
Risk ranking
Recently, a promising method, named risk ranking, has been recommended by the Codex Alimentarius Commission (CAC) (52), which can be used to assess identified hazards; in this case, risk of contaminants in foods evaluate by scoring the probability of risk according to a set of variables. Each variable has a risk category from severe to low, and scores are calculated based on the likely outcome according to frequency data, the contaminants levels, potential repercussions, and the published data in the literature that have described the contaminant.
Risk ranking of a contaminant helps risk managers to set priorities in food safety issues from accepting a risk (if relatively low) through to mitigating risks. Several criteria with their scoring guidance, including toxicities, risk control difficulties, severities of risk, brand reputation in society, a maximum level of detection, and rates of detection, have been established by CAC for risk ranking (Table 4). Accordingly, the scores for a food contaminant can be calculated using formula 3 as described by CAC (52):
Overall score = first index score × (second index score + third score index + fourth score index + fifth score index + sixth score index)
Equation 4.
The overall score of a particular hazard, in this case, food contaminants, calculated by the risk ranking method, is then classified into four categories, including low risk, medium risk, relatively high risk and high risk with overall scores of <50 (low risk); 50-75 (medium risk); 75-100 (relatively high risk); >100 (high risk).
To date, to the best of our knowledge, very little information is available in the literature on using the risk ranking approach mentioned above. However, when applied to the hazard of aflatoxins in cereal-based baby foods, this risk ranking method categorized the presence of AFB1 as a high risk for babies that consume cereal-based baby food, as AFB1 overall score was 110, which classifies AFB1 into high-risk group (Table 5).
Furthermore, the overall risk ranking score for AFG1 was 60, indicating a moderate risk due to the presence of AFG1 in cereal-based baby food for babies aged >24 months. Regarding the overall risk scores for AFB2 (28) and AFG2 (22), these aflatoxins can be classified as low-risk contaminants in cereal-based baby food for babies aged >24 months.
The results of the risk ranking of the AFs when taken together with the estimated dietary exposure, margins of exposure and the potential risk of cancer to AFs through the rates of consumption of cereal-based baby food, indicates that the presence of AFB1 in cereal-based baby foods is a serious health risk for babies and demands the attention of risk managers to reduce or eliminate this risk for the most vulnerable sector of society.
Management advises
The infant food producers are advised to put the importance of mycotoxins content in their priorities. Enough information and training must be provided for manufacturers, parents, and health care professionals to reduce the health risk associated with mycotoxins, particularly aflatoxins. It is vital to keep the levels of contaminants low in order to secure public health. Eventually, inspection and surveillance should be constant, extensive, and must be carried out by the government and related ministries because the final product quality depends on accurate control at every step of the manufacturing process.
| no | Cold season samples | Warm season samples | ||||||
|---|---|---|---|---|---|---|---|---|
| AFB1 | AFB2 | AFG1 | AFG2 | AFB1 | AFB2 | AFG1 | AFG2 | |
| 1 | 0.04 | 0.01 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 2 | 0.5 | 0.04 | <LOQ | <LOQ | 0.38 | 0.04 | <LOQ | <LOQ |
| 3 | 0.09 | 0.01 | <LOQ | <LOQ | 0.09 | 0.01 | <LOQ | <LOQ |
| 4 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 0.01 |
| 5 | <LOQ | <LOQ | <LOQ | <LOQ | 0.05 | <LOQ | <LOQ | <LOQ |
| 6 | 0.84 | 0.08 | <LOQ | 0.02 | 0.7 | 0.08 | <LOQ | 0.02 |
| 7 | 0.51 | 0.05 | <LOQ | 0.02 | 0.3 | 0.03 | <LOQ | 0.01 |
| 8 | 0.14 | 0.02 | <LOQ | 0.08 | 0.15 | 0.02 | <LOQ | 0.08 |
| 9 | <LOQ | <LOQ | <LOQ | 0.02 | <LOQ | <LOQ | <LOQ | 0.02 |
| 10 | <LOQ | <LOQ | <LOQ | <LOQ | 0.15 | 0.01 | <LOQ | 0.01 |
| 11 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 12 | 0.05 | 0.01 | <LOQ | <LOQ | 0.17 | 0.02 | <LOQ | <LOQ |
| 13 | <LOQ | 0.01 | <LOQ | <LOQ | 0.05 | 0.01 | <LOQ | <LOQ |
| 14 | <LOQ | <LOQ | <LOQ | <LOQ | 0.04 | 0.01 | <LOQ | <LOQ |
| 15 | 0.28 | 0.03 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 16 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 17 | <LOQ | 0.01 | <LOQ | <LOQ | 0.31 | 0.03 | <LOQ | <LOQ |
| 18 | 0.04 | 0.01 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 19 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 20 | 0.11 | 0.03 | <LOQ | <LOQ | 0.06 | 0.02 | <LOQ | <LOQ |
| Mean | 0.13 | 0.0155 | <LOQ | 0.007 | 0.1225 | 0.014 | <LOQ | 0.0075 |
| Age groups | AFB1 | Total AFs | ||||
|---|---|---|---|---|---|---|
| P5 | P50 | P95 | P5 | P50 | P95 | |
| 6-12 | 0.05 | 0.54 | 5.81 | 0.32 | 0.82 | 8.55 |
| 12-18 | 0.04 | 0.46 | 4.96 | 0.27 | 0.60 | 11.30 |
| 18-24 | 0.04 | 0.41 | 4.39 | 0.25 | 0.55 | 9.69 |
| Age groups | AFB1 | Total AFs | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P5 | P50 | P95 | P5 | P50 | P95 | |||||||||||
| MoE | Cancer | MoE | Cancer | MoE | Cancer | MoE | Cancer | MoE | Cancer | MoE | Cancer | |||||
| 6-12 | 69.5 | 0.0007 | 731.7 | 0.0008 | 7814.7 | 0.08 | 0 | 0.0047 | 588.7 | 0.012 | 1208 | 0.12 | ||||
| 12-18 | 81.5 | 0.0005 | 861.7 | 0.0006 | 9102.2 | 0.07 | 0 | 0.0040 | 695.3 | 0.008 | 1416.2 | 0.16 | ||||
| 18-24 | 90.4 | 0.0005 | 964.4 | 0.0006 | 10351.8 | 0.06 | 0 | 0.0037 | 778.1 | 0.008 | 1581.4 | 0.14 | ||||
| Index | Index value | Index value | Index value | Index value |
|---|---|---|---|---|
| Toxicity | High | Relatively high | Medium | Low |
| Degree of difficulty in risk control | Difficult | Poor | Potentially poor | Capable |
| Severity | Serious | Relatively serious | Medium | Noteworthy |
| Social reputation | Serious | Relatively serious | Medium | Noteworthy |
| Maximum amount of detection residue (μg/kg) | >5000 | 1000-5000 | 500-1000 | 0-500 |
| Detection rate*% | >10 | 8-10 | 6–8 | 4–6 |
| Risk factors | Toxicity | difficulty in risk control | Severity | Social reputation | Detection residue | Detection rate (%) | Overall score | Risk |
|---|---|---|---|---|---|---|---|---|
| AFB1 | High | Difficult | Serious | Serious | 0-500 | >10 | 110 | High |
| AFB2 | Low | Potentially poor | Medium | Noteworthy | 0-500 | >10 | 30 | Low |
| AFG1 | Relatively high | Potentially poor | Relatively serious | Relatively serious | 0-500 | 0 | 60 | medium |
| AFG2 | Low | Potentially poor | Medium | Noteworthy | 0-500 | 4-6 | 24 | low |
Conclusion
Aflatoxins (AFs) occurrence in cereal-based foods is a global health concern as they are used worldwide as complementary foods in babies’ diets. Results showed an occurrence ranging 20% to 60% for B1, B2, and G2 aflatoxins in cereal-based baby foods in Iran, while AFG1 was not detected. 10% and 30% of total samples were exceeded the maximum limit of AFB1 (0.5 ng/g) established by National Standard Organization of Iran and European Commission maximum (0.1 ng/g), respectively. The Monte Carlo simulation model showed a significant difference in estimated dietary exposure among three assessed age groups (6-12 months, 12-18 months, and 18-24 months) with the highest estimated dietary exposure to both AFB1 and total AFs for 6-12 months aged babies. In general, except in one case, a MoE lower than 10,000 was estimated by Monte Carlo simulation approach for all 5, 50, and 95 percentiles in all the age groups to AFB1 and total AFs, indicating a health concern about AFB1 and total AFs exposure through cereal-based baby food consumption from Iran in children aged <24 months. For the AFB1 and TAFs analyzed, a high cancer risk for high consumers (P95) of cereal-based baby food aged <24 months was also estimated, calling for immediate intervention due to serious claims that AFB1 is highly carcinogenic, inducing hepatocellular carcinoma. According to the risk ranking results, the presence of AFB1 is a high risk for babies who consume cereal-based baby food, which demands the attention of risk managers to reduce or eliminate this risk for the most vulnerable sector of society; however, the presence of AFG1, AFB2 and AFG2 were classified as moderate risk, low risk, low risk, respectively, in cereal-based baby foods for baby consumers whose aged <24 months.