Introduction
Cutaneous leishmaniasis (CL) is considered a protozoan zoonotic disease induced by protozoa of the genus Leishmania and transmitted by sandflies to various mammal hosts, including humans. CL has an annual incidence of 0.6 to 1 million cases, with a mortality rate of 60000 per year (1). However, disease incidents are increasing worldwide nowadays. CL causes by over 17 Leishmania species, in which old-world L major species are the foremost causes of acute CL. CL therapy’s standard drug is the first-line drug of pentavalent antimonial (Sbv), meglumine antimoniate (glucantime), and the second drug line, pentamidine amphotericin B, which used for over the years, and they show adverse reactions and toxicity (2, 3).
Herbal product-derived medication is an encouraging potential source for treating parasitic diseases such as artemisinin, a sesquiterpenoid lactone compound in malaria. Xanthatin is also natural sesquiterpene lactones isolated from Xanthium strumarium L., an annual herb that belongs to the family Asteraceae genus Xanthium (4, 5). Sesquiterpenoid lactone, which is also called xanthanolides, is an active component of these medicinal plants and is traditionally used to remedy several ailments in many countries (6). Xanthatin with formula C15H18O3 and molecular weight of 246.3 possesses remarkable anti-proliferative activity against various tumor cells in-vitro and in-vivo through downregulating the STAT3, GSK3B, and Beta-catenin (6-9). It also triggered ChK1-mediated DNA damage and destabilized Cdc25C via lysosomal degradation. Nibret et al. described the anti-inflammatory activity of xanthatin by inhibiting both PGE2 synthesis and 5-lipoxygenase activity (10). Xanthanolides possess antimicrobial, anti-protozoal, and cytotoxic activities (11). Lavault et al. described the leishmanicidal actions of xanthanolides isolated from Xanthium macrocarpum DC. They recorded that xanthinin was the most active xanthanolides toward L. infantum and L. Mexicana, with xanthatin against L. infantum (12).
The recent report on macrophages’ immune metabolism has shown metabolic repolarization as an attractive therapeutic target for disease conditions (13). The concept of unlike phenotypes of classically (M1) and alternatively (M2) activated macrophages was initially being proposed by Mills (14), and Thapa and Lee (15) explained its biochemical pathway signature variations. It postulated that macrophage’s metabolism could shift from an aerobic (with oxygen) profile based on oxidative phosphorylation to an anaerobic (without oxygen) one based on glycolysis and vice-versa (16). These metabolome profiles provide energy support for immune activity and directly affect immune cell functions by controlling transcriptional and post-transcriptional events in macrophages’ immunity, leading to destroying pathogens or being sacrificed (16).
Recent advances in metabolomics are opening up new horizons for understanding the metabolites signals that regulate “immunity” (17). Metabolomics is a new field of studying the metabolome profiling of a living organism with nuclear magnetic resonance spectroscopy. In a series of investigations, we consider the leishmanicidal activity of the various xanthanolides fraction of X. Strumarium in CL. The present study aimed to determine the effect of xanthatin on L major amastigotes and mice macrophage’s metabolism on L. major’s growth and viability in cell-free culture and macrophages amastigotes.
Experimental
Plant material
X. strumarium leaves obtained from Kermanshah Province, Iran. A voucher specimen was deposited in the central herbarium of Tehran University, Tehran (number 48241).
Preparation of plant crude extracts
The air-dried leaves of X. strumarium (4 kg) were macerated with an Ethanol-water solution (80%) at room temperature for 72 h. The resulting extract was mixed with active charcoal, centrifuged to isolate plant pigments. This mixture was filtrated and concentrated under reduced pressure in rotavapor at 45 °C to obtain a dark brown syrup (450 g) (18).
Isolation and characterization of xanthatin
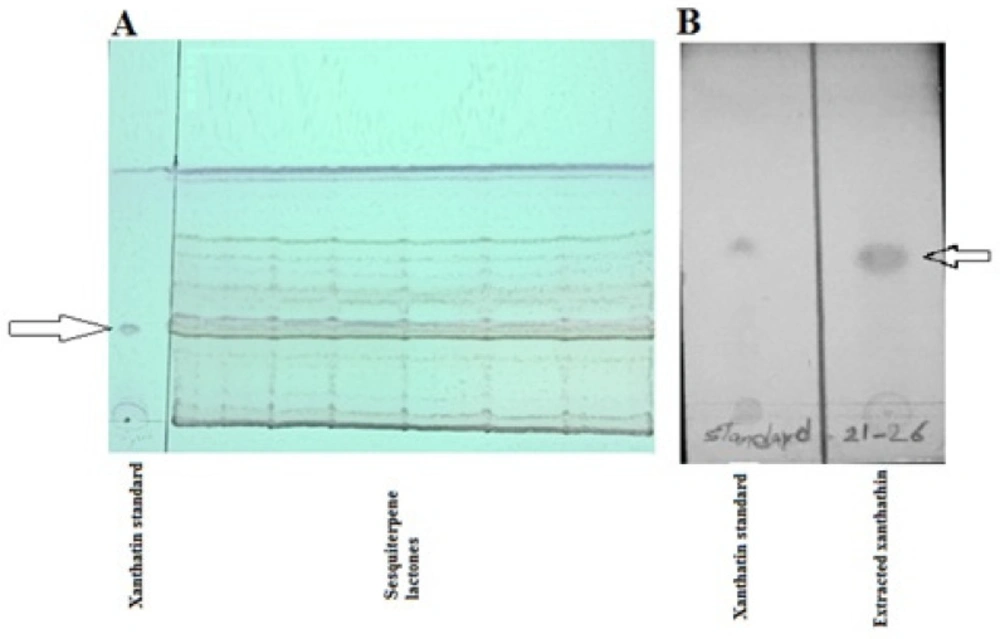
Xanthatin was isolated, as described by Nibret et al. (10). In brief, 450 g of the residue obtained was mixed with 200 g silica gel and dried at 40 °C. The mixture was placed onto the top of the silica gel column chromatography (50 × 3 cm). The fractions were collected manually every 30 min after adding 100% cyclohexane followed by cyclohexane: ethyl acetate 9:1 (V/V) to 100% ethyl acetate in a 10% stepwise ratio to raise the solvent polarity. Totally 38 tubes were collected, all tube’s content was analyzed by thin-layer chromatography (TLC) under ultraviolet (UV) light along with xanthatin standard (ChemFaces CAS No. 26791-73-1). Based on the TLC results profile, xanthatin fraction eluted after cyclohexane: ethyl acetate 1:9 ratio passed through the column as mobile phases. Pooled fractions (tubes 21-26) were recrystallized with methanol to obtained white needle crystal as xanthatin. This procedure was repeated to collect adequate xanthatin (15 mg). Xanthatin identity was confirmed by NMR (Bruker-500 MHz), and its purity was checked by Nibret’s method (10, 19).
Cell culture
A Blab/c mice macrophage cell line (J774) was cultured in RPMI 1640 medium (Atocel), containing 10% FBS (Gibco), Penicillin100 U/mL - Streptomycin 100 µg/mL (Sigma) as antibiotics at 37 °C with 5% CO2.
Parasite culture
The isolated amastigote of L. major (strain MRHO/IR/75/ER) from the lymph node of infected BALB/c mice was grown in M199 medium (Sigma, Germany) supplemented with 10% FBS, Penicillin100 U/mL - Streptomycin 100 µg/mL, two mM L-glutamine, 0.1 mM adenosine, 0.5 μg⁄mL hemins, and 40 mM HEPES at 23-26 ºC for 5-6 days. The stationary phase of promastigotes was centrifuged at 3000 rpm for 10 min at 4 °C and used to infect J774 macrophages (20).
Preparation of xanthatin
Ten and half milligram of xanthatin dissolved in 1 mL DMSO 1% and M199 medium (without FBS), sterilized by 0.22 μm micro-filters as stock solution and kept at 4 °C until use.
Macrophage toxicity assay
J774 macrophages (8 × 103cell/well) were seeded in every well of 96-well culture plate (in triplicates) and incubated in RPMI 1640 supplemented with 10% FBS 37 ºC with 5% CO2 to attach. After 24 h, all wells’ medium was replenished with different xanthatin fractions (1050 to 0.007 µg/mL). The wells with no xanthatin (only cells and medium) and the wells without cells and xanthatin were considered negative control and blank sequentially. Also, Amphotericin B was used as positive control. The plate was incubated at 37 °C for 48 h. After adding 20 µL thiazolyl blue tetrazolium bromide (MTT) reagent (5 mg/mL in PBS) into each well, the plate was kept at 37 °C until the purple formazan crystals were visible under a microscope after 4 h. All medium was then discarded, and added 100 µL of DMSO into each well, including controls, shacked gently to dissolve formazan crystals and observe purple. Every well’s absorbance was recorded at 570 nm against 630 nm as a reference using a plate reading spectrophotometer. The formula Below determined the value of 50% inhibitory concentrations (IC50) and Percentage macrophage viability (Equation 1) (21):
Macrophage infection
6× 104 cells of J774 macrophages seeded in 24-well culture plates equipped with 10 mm coverslips. The coverslips containing cells were incubated 24 h to adhere in the M199 medium at 37 °C with 5% CO2. Then, non-adherent cells were washed with phosphate-buffered saline (PBS) and infected with a stationary phase of L. major (10:1 parasite/macrophage) at 37 °C 5% CO2 for 4 h. Non-internalized promastigotes were washed with PBS (4 times), and the plate was incubated again in M199 medium supplemented with 2% FBS for 24 h (21, 22).
Anti-amastigote assay
Axenic amastigotes were treated in triplicates by various concentrations of xanthatin (353.75 to 0.0007 mg/mL) once. The three wells, amastigotes without any xanthatin, considered negative controls, and amphotericin B, was used as a positive control to compare the efficacy of xanthatin. All M199 medium and xanthatin concentrations were replenished every 24 h for three days. Then, wells were washed with PBS, fixed with methanol, and stained with 10% Giemsa. The infection rate (IR Equation 2) and multiplication index (MI, Equation 3) in each stained coverslip were determined microscopically and calculated. Using two Formulas Below (21, 22):
Treatment of amastigotes with xanthatin
The number of 4 × 107 J774 macrophages (in each 175 cm cell culture flask) infected with 4 × 108 stationary promastigotes of L.major (5 replicates experimental and five replicates as negative control). All samples were incubated in an M199 medium containing 2% FBS at 37 °C with 5% CO2 for 24 h. Based on the xanthatin IC50 value, every experimental flask was treated with 1.575 µg/mL of xanthatin. The medium was replenished daily for three days in every flask. After five days, cells in each flask were washed once in PBS 1X (pH 7.4) gently, trypsinized, and transferred to 50 mL falcon tubes (21).
Cell quenching and metabolites extraction for NMR analysis
All falcons were centrifuged at 4000 rpm at 4 °C for 15 min. Each pellet (containing 4 × 107 amastigotes, approximately) was washed using ice-cold PBS (three times) to remove any impurities and residual media. The quenching process was done by tubes immersing into liquid nitrogen until freezing and thawing in an ice bath (0 °C) (23). The cell extraction process for 1HNMR spectroscopy was done on quenched cells in each tube. In brief, Ice-cold 1.8 M perchloric acid (200 µL/106 cells) was added, followed by 5 min the sonication in an ice bath to disrupt the amastigotes membrane. All samples were kept in an ice bath for one hour to allow the precipitation of potassium perchlorate. After centrifugation at 12000 rpm at 4 °C for 10 min, the supernatant’s pH adjusted to 7.2, the samples were lyophilized and stored at −80 °C until future analysis (24).
1HNMR Spectroscopy
Lyophilized sample (n = 10) dissolved in 600 µL phosphate D2O buffer (100 mM, pH 7.2) containing 1 mM trimethylsilyl propionate (TSP) as a chemical shift reference (δ = 0 ppm) and two mM imidazole as pH indicator (δ = 5.50 to 8.80 ppm). Finally, samples were centrifuged at 18000 rpm for 15 min at 4 °C, and 500 µL supernatants were transferred into an NMR probe.
NMR spectra of samples were obtained at 500.13 MHz for proton observation at 298K using a Bruker AV-500 NMR spectrometer. One-dimensional 1HNMR spectra were recorded using a 10-µs pulse, 0.1 s mixing time, 3.0 s relaxation delay, 6009.6 Hz spectral width, and 3000 transients with standard 1D NOESY pulse sequence to suppress the water peak (23, 24).
Data Analysis
1HNMR spectra were pre-processed by applying custom-written ProMatab (V.3.3) code in MATLAB (v.7.8.0.347) to convert spectra into a suitable format for multivariant analysis. Spectra binned into 0.005 ppm chemical shift, and water peak at 4.7 ppm eliminated. Data normalization and Pareto scaling were performed before data classification. A supervised Partial Least Square- Discriminate Analysis (PLS-DA) classification technique was used to identify notable outliers between experimental groups. Human Metabolome Database (HMDB) and LeishCyc databases were utilized to discover the corresponding metabolites to spectral outliers. Metabolic pathways worked out by using online MetaboAnalyst Ver. 3.0 (www.Metaboanalyst.ca) a metabolomics analysis software and SPSS to determine the p values.
Results
Isolation and identification of xanthatin
Adequate quantities of xanthatin (15 mg) were collected by column chromatography, followed by TLC techniques (Figure 1). Crystalized xanthatin was analyzed with NMR spectroscopy, and the spectra were in agreement with xanthatin NMR spectra provided along with standard by ChemFaces company and also Marco JA et al. (25)
Cytotoxicity effect of Xanthatin on Macrophages J774
Cell viability results of different xanthatin concentrations on J774 macrophages are described in Table 1. Cell viability was raised with decreases in the stock extract concentration and xanthatin IC50 defined as 0.75 µg/mL. Nevertheless, xanthatin with our stock concentration (1050 µg/mL) showed the highest cytotoxic activity (100%).
The results for the activity of xanthatin and amphotericin B on amastigotes are depicted in Table 2. Based on xanthatin concentration (0.5 µg/mL), the rate of IR and MI of xanthatin was estimated at 53% and 62.5%, respectively, and this concentration was chosen for our further metabolomics NMR study.
1H- NMR spectroscopy analysis
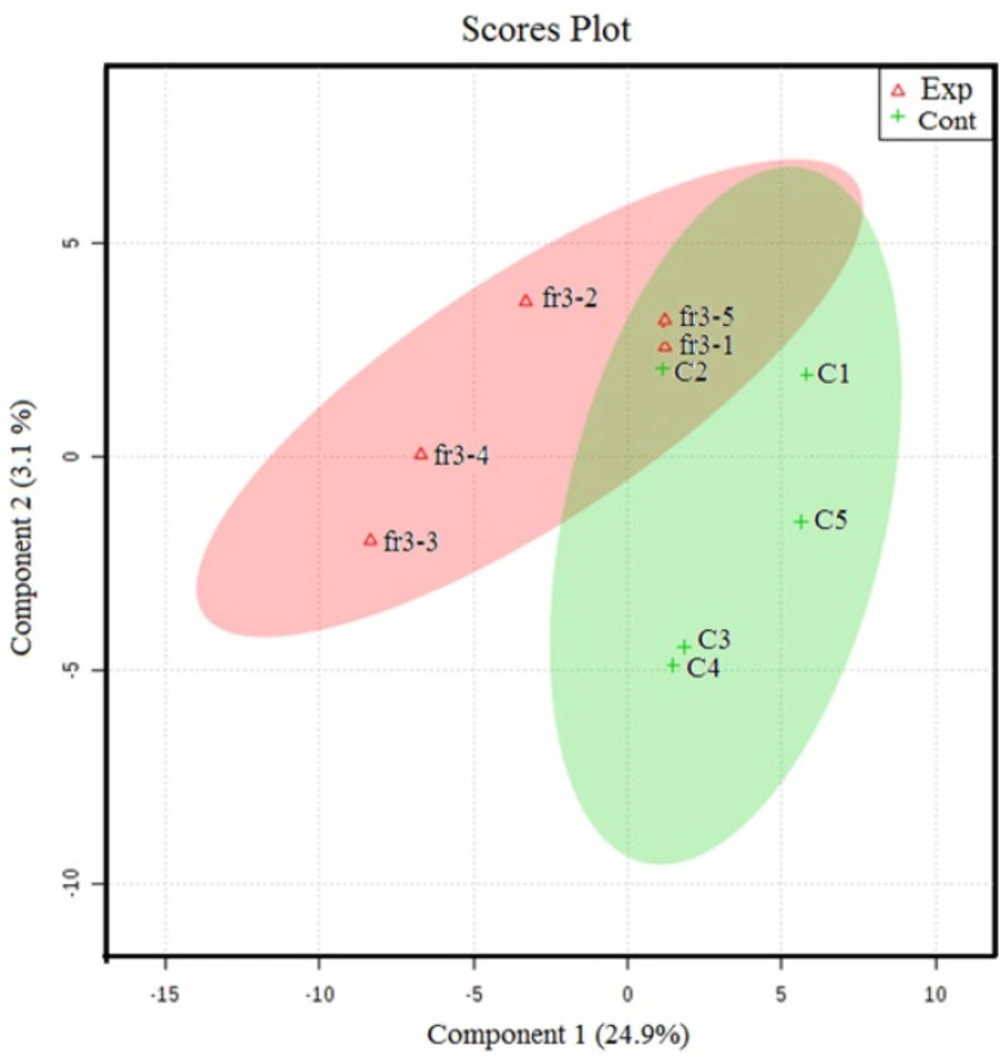
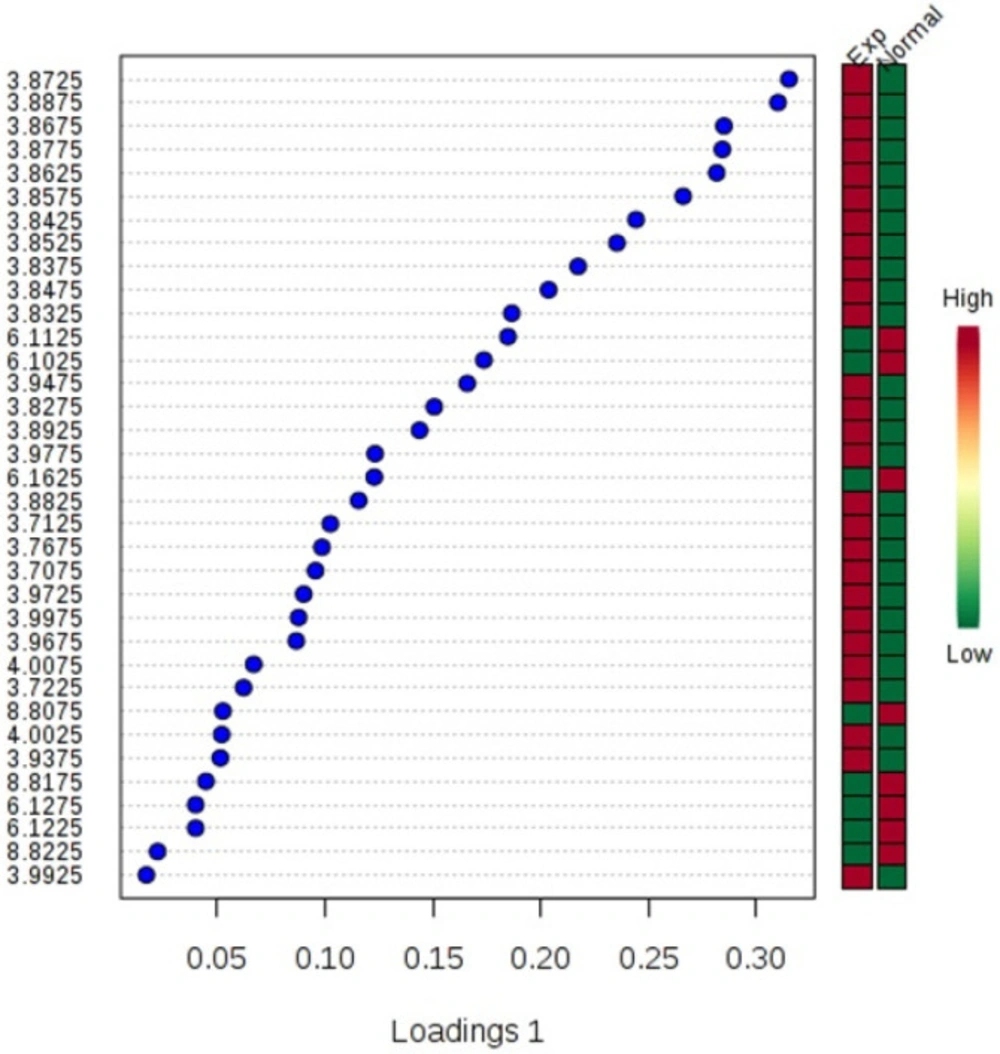
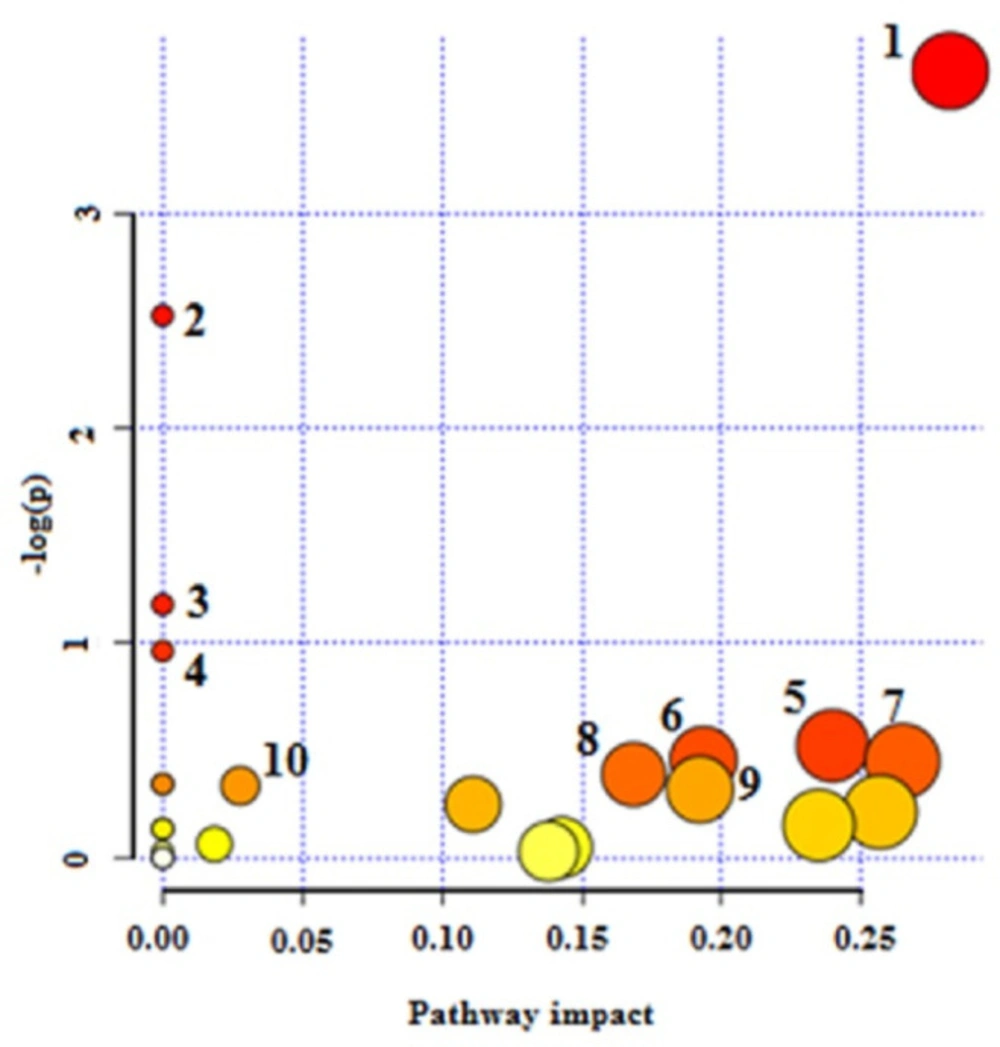
The NMR spectroscopy analysis revealed a spectral peak variation in two groups of experiments; changes are more confined to regions 3, 6, and 8 ppm of spectra. PLS-DA score plot revealed that both groups had accurately classified (Figure 2), and the VIP plot explicated the chemical shift dimension (Figure 3). Most modified metabolites showed in Table 3, and the metabolic pathways analysis degree of centrality is described in Figure 4. The detail of the pathway’s analysis showed in Table 4. According to a statistical p-value of pathways value, less than 0.05 was taken for discussion in this article.
Discussion
Pentavalent antimonial, i.e., amphotericin B, pentamidine, Miltefosine, and paromomycin, are known as efficient drug medications leishmaniasis therapy (26). Several mechanisms of action are proposed for these drugs; however, for a vast reason, like; host immune system, antileishmanial pharmacokinetics, and leishmania factors, their efficacy and drug resistance observed in leishmaniasis (27, 28).
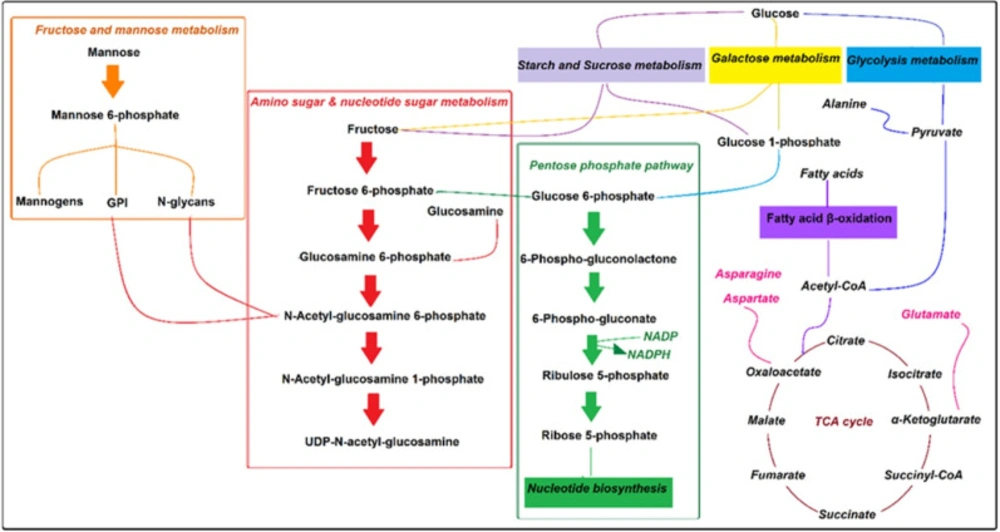
Fatty acid uptake is the primary carbon source in amastigotes. Acetyl- CoA is produced by glycosomal pyruvate and fatty acid β-oxidation process required for the Krebs cycle to produce energy (29, 30). Therefore, the main drug target of pentavalent antimonies is based on the inhibition of Leishmania 𝛽-oxidation fatty acids and glycolysis pathway (26, 31). There are also records that the antileishmanial activity of Miltefosine is related to phospholipid biosynthesis lipid pathways and cytochrome C oxidase (26, 32). It is also stated that essential amino acids in amastigotes are usually utilized for protein synthesis (Figure 5), and paromomycin other antimony interferes with protein biosynthesis in the parasite (26, 27).
Amphotericin B binds to membrane sterols and phospholipids and kills the parasite by altering membrane permeability, cellular potassium, magnesium, glucose, and water disbalance (26). Researchers declare that inferences including; up-regulation of glycolysis and the TCA cycle (33), changes in membrane fluidity and strolls composition, augmentation of membrane MDR1 pumps, and Drug Efflux, reducing reactive oxygen species and thiol, leading to drug amphotericin B resistance (26). Based on our results, as the strength of anti leishmanicidal activity of drugs maters, xanthatin may show equal potency compared to amphotericin B, and the affected pathways are confined to amino sugar and nucleotide sugar metabolism, starch and sucrose metabolism, cyanoamino acid metabolism, Galactose metabolism, and pentose phosphate pathway only.
Generally, Leishmania’s energy demand is provided by carbon transformation in glycolysis, Krebs cycle, gluconeogenesis, and pentose phosphate pathway, which is called central carbon metabolism. It is believed that the living and prime proliferation of Leishmania amastigotes in mammalian hosts depend on phagolysosome metabolism.
Various types of sugars in phagolysosomes have emerged from the turnover of extracellular matrix compositions such as host glycoproteins, glycosoaminoglycans, and proteoglycans (34). Also, several genes related to hexosamines metabolism are reported in Leishmania genomes (35).
Naderer et al. showed that scavenged hexosamines are vital sugars in leishmania phagolysosomes. Their catabolism is necessary to maintain critical biochemical pathways and prevent hexosamine toxicity (36).
Malcolm J. et al. postulated that some phosphorylated hexosamines produce surface glycoconjugates like GPI and mannogens in glycosomal glycolysis and pentose phosphate pathways (30). So, these pathways play essential roles in the pathogenesis of amastigotes.
Based on statistical significance (p-value ≤ 0.05) within our findings, some hexosamines metabolites like; D-glucosamine 6-phosphate, N-Acetyl-alpha-D-glucosamine in amino sugar, and nucleotide sugar metabolism were the most changed metabolites.
Amino sugars pathway in L. major amastigotes is utilized as sources of carbon and energy (36). Also, enough a concentration of amino sugars like glucosamine and N-acetyl glucosamine is needed to maintain the lowest hexosamines in the phagolysosome. They are transported to the glycosome and, after phosphorylation, convert to fructose-6-phosphate by some enzymes such as glucosamine 6-phosphate deaminase (GND).
Former results demonstrated that in a situation in which hexosamines are considered the primary source of carbohydrate, glycosomes of the Leishmania parasite could be targeted by GND, which plays an essential role in the growth and development of leishmania infection in susceptible animals (36).
Fructose-6-phosphate is a crucial metabolite in glycoconjugate biosynthesis, and it is finally exported to the cytoplasm to produce compositions like mannogens and N-glycan. Our results showed that treated amastigotes by xanthatin exhibited some variations in hexose metabolites such as alpha-D-glucose, glucose 1-phosphate, beta-D-fructose, fructose 6-phosphate, and mannose 6-phosphate.
Our results are then approved by earlier studies and suggest that any variation in the first metabolites of the amine sugar pathway or GND results in subsequent metabolite alterations of parasites. Moreover, our findings revealed that some metabolites, including; beta-D-fructose, alpha-D-glucose, and glucose 1-phosphate, have changed in starch and sucrose and Galactose metabolism pathways. Thus, it seems that the central role of xanthatin in amastigotes metabolism might have been performed by these critical metabolites, although further investigation should conduct to confirm this statement.
Some studies reported that the rate of amino acid oxidation elevates in L.major amastigotes. Amastigotes de novo pathway synthesizes non-essential amino acids like aspartate, alanine, and glutamate. On the contrary, with promastigotes, fatty acids have an essential role in de novo biosynthesis in phagolysosomes (34). Intracellular amastigotes are dependent on fatty acid β-oxidation and Krebs cycle for energy production. It is also stated that acetyl-CoA result from fatty acid β-oxidation is expensed in the Krebs cycle to produced intermediates for biosynthesis of non-essential amino acids, and they are used for biosynthesis of amino sugars, nucleotides, and thiol in amastigotes (36-38). The viability and virulence of amastigotes in phagolysosomes effect via disruption of key enzymes in the Krebs cycle for non-essential amino acid biosynthesis (29), because of the inhibition of some genes and proteins involved in β-oxidation and mitochondrion respiratory chain (39, 40). Our metabolomics-based study also revealed that the amastigote concentration of aspartate and asparagine amino acids in cyanoamino acid metabolism has changed as two targets for xanthatin.
Glucose 6-phosphate and 6-phosphogluconate in the pentose phosphate pathway are catalyzed by oxidative branch enzymes, including glucose-6-phosphate dehydrogenase, 6-phosphogluconate dehydrogenase to produced Ribose 5-phosphates and NADPH. Ribose 5-phosphates needed for DNA and RNA, another nucleotide biosynthesis (41), and NADPH plays a crucial role in antioxidant defenses against Leishmania species. Several studies focused on the pentose phosphate pathway role and its enzymes in some trypanosomes and Leishmania species ( (42, 43). Mehlotra et al. declared that there are not enough antioxidant enzymes available in Leishmania promastigotes, so they are sensitive to oxidative stress (44), and Deschacht focused on the relationship between oxidative stress mechanism and Leishmania infection (45). Moreover, Mukherjee et al. postulated that amphotericin B could affect macrophages’ antioxidant system (46, 47). Therefore, some researchers concluded that the pentose phosphate pathway could be considered as drug target for the treatment of leishmaniasis (43, 48). Our research revealed that Beta-D-glucose 6-phosphate, 6-D-phosphogluconate, and D-ribose 5-phosphate in the pentose phosphate pathway varied significantly between the two groups of study. Hence, our finding is commonly in line with prior reports.
Pathway analysis degree of centrality. 1-Amino sugar and nucleotide sugar metabolism 2- Starch and sucrose metabolism 3- Cynoamino acid metabolism4- Galactose metabolism 5- Pentose phosphate pathway 6- Glycerolipid metabolism 7- Alanine, aspartate, and glutamate metabolism 8- Fructose and mannose metabolism 9- Pentose and glucuronate interconversions and 10- Valine, leucine, and isoleucine biosynthesis
| Concentrations. ( | Xanthatin IC50 | Amphotericin B. IC50 |
|---|---|---|
| 1050 | 4.3 % | 0% |
| 525 | 5.1 % | 0.1% |
| 105 | 7.0% | 2.1% |
| 52.5 | 8.8% | 2.3% |
| 10.5 | 10.2% | 8.6% |
| 5.25 | 24.3% | 19.2% |
| 1.5 | 34.5% | 28.8% |
| 0.75 | 53.7% | 49.3% |
| 0.15 | 57.6% | 51.4% |
| 0.075 | 66.4% | 65.9% |
| 0.015 | 70.4% | 76.5% |
| 0.0075 | 75.2% | 82.3% |
In-vitro activity of xanthatin on Macrophages
| Multiplication rate (%) | Infection rate (%) | Xanthatin Concentration (µg/mL) |
|---|---|---|
| 0% | 0% | 1050 |
| 10.2% | 9% | 5 |
| 23.5% | 20% | 3 |
| 48.0% | 41 % | 1 |
| 62.5% | 53% | 0.5 |
| 71.4% | 61% | 0.2 |
| 74.1% | 63% | 0.1 |
| 88.2% | 75% | 0.05 |
| 100% | 90% | 0.02 |
| 100% | 85.3% | Negative control |
The activity of xanthatin on amastigotes
| Metabolite name | Metabolite name | Metabolite name | Metabolite name |
|---|---|---|---|
| 1,3-Butanediol | 1,5-Anhydrosorbitol | 1-Methylguanosine | D-Aspartic acid |
| 2,3-Butanediol | 2,4,5 Trimethoxybenzaldehyde | 2-Methylbutyl acetate, | dCMP |
| 3 Stachyose | 3-Hydroxydodecanedioic acid | 3-Methoxy-4-Hydroxyphenylglycol sulfate | Dehydroascorbic acid |
| 3-Hydroxysebacic acid | 3-Hydroxytetradecanedioic acid | 3-Hydroxydodecanoic acid | Deoxyadenosine |
| 3-Methoxytyrosine | 3-Nitrotyrosine | 3-Phosphoglyceric acid | Deoxyinosine |
| 6-Phosphogluconic acid | 7-Methylguanosine | Adenosine | Deoxyuridine |
| Adenosine monophosphate | Allocystathionine | Allose | Dethiobiotin |
| Alpha-D-Glucose | Alpha-Lactose | Atenolol | D-Fructose 2,6-bisphosphate |
| Azacitidine | Beta-D-Glucose 6-phosphate | Beta-N-Acetylglucosamine | D-Fructose |
| Cellobiose | Chlorogenic acid | Cytidine monophosphate | D-Galactose |
| Cytidine monophosphate | D-Glucose | Diethylthiophosphate | DL-Homocysteine |
| DL-O-Phosphoserine | D-Maltose | D-Mannose | D-Ribose 5-phosphate |
| D-Serine | D-Tagatose | Enilconazole | Ethenodeoxyadenosine |
| Flavin Mononucleotide | Fructose 6-phosphate | Galabiose | Galactonic acid |
| Galactose 1-phosphate | Galacturonic acid | Gluconolactone | Glucosamine 6-phosphate |
| Glucosamine 6-sulfate | Glucose 1-phosphate | Glucose 6-phosphate | Glyceric acid |
| Glycerol 3-phosphate | Glycerophosphocholine | Guaifenesin | Homocysteine |
| Hydroxypropionic acid | Inosine | Isomaltose | Isopropyl alcohol |
| Isopropyl alcohol | Isovalerylcarnitine | L-Alpha-aminobutyric acid | L-Arabinose |
| L-Arabitol | L-Asparagine | L-Aspartic acid | L-Cystathionine |
| L-Cystine | L-Gulonolactone | L-Hexanoylcarnitine | L-Histidine |
| L-Histidinol | L-Homocysteine | L-Homoserine | L-Iditol |
| L-Leucine | L-Octanoylcarnitine | L-Palmitoylcarnitine | L-Serine |
| L-Sorbose | Maltitol | Maltotetraose | Maltotriose |
| Mannitol | Mannose 6-phosphate | Melibiose | Methionine sulfoxide |
| Muramic acid | N-Acetylgalactosamine 4-sulphate | N-Acetylgalactosamine | N-Acetyllactosamine |
| N-Acetylmannosamine | N-Acetylneuraminic acid | N-Acetylserine | Neopterin |
| O-Phosphoethanolamine | Orotidine | Pseudouridine | Quinic acid |
| Uridine diphosphate-N-acetylglucosamine | Riboflavin | Ribonolactone | Sedoheptulose |
| Shikimic acid | Sorbitol | Sucrose | Thiamine |
| Threonic acid | Thymidine | Trehalose | Uridine 5'-monophosphate |
Metabolites affected by xanthatin
| Pathway | Total | Hits | -Log (p) | FDR | Metabolites |
|---|---|---|---|---|---|
| Amino sugar and nucleotide sugar metabolism | 21 | 8 | 0.00257 | 1.00 | -D-Glucose 1-phosphate; -α-D-glucose; D-Mannose; Fructose 6-phosphate; D-Glucosamine 6 phosphate: -beta-D-fructose: -Mannose 6-phosphate : α-D-Glucosamine: UDP-N-Acetyl-alpha-D-glucosamine |
| Starch and sucrose Metabolism | 6 | 3 | 0.00803 | 1.00 | α-D-glucose; beta-D-fructose; D-Glucose 1-phosphate |
| Cynoamino acid metabolism | 6 | 2 | 0.0308 | 1.00 | L-aspartate; asparagine - |
| Galactose metabolism | 7 | 2 | 0.0593 | 1.00 | α-D-glucose; D-Glucose1-phosphate |
| Pentose phosphate pathway | 16 | 3 | 0.0593 | 1.00 | Beta-D-Glucose 6-phosphate; 6-D-phosphogluconate; D-Ribose 5-phosphate |
| Glycerolipid metabolism | 11 | 2 | 0.0634 | 1.00 | D-Glycerate; Glycerol 1-phosphate |
| Alanine, aspartate, and glutamate metabolism | 17 | 3 | 0.0709 | 1.00 | L-Aspartate; L-Asparagine; D-Glucosamine 6-phosphate |
| Fructose and mannose metabolism | 18 | 3 | 0.0678 | 1.00 | D-Mannose; D-Mannose 6-phosphate; Trimethylamine |
| Pentose and glucuronate interconversions | 6 | 1 | 0.0709 | 1.00 | D-Glucose 1-phosphate |
| Valine, leucine, and isoleucine biosynthesis | 6 | 1 | 0.0709 | 1.00 | L-Leucine |
Pathway analysis detail
Conclusion
Currently, there is a strong trend in herbal medicine for the treatment of leishmaniasis worldwide. To date, several research studies have focused on the therapeutic effects of XanthiumStrumarium extracts on leishmaniasis. This present pilot study was conducted for the first time to survey the antileishmanial activity of xanthatin and its impact on macrophages and amastigotes’ metabolism. Our finding suggests that the main target of xanthatin in amastigotes is carbon metabolism, which is an essential step for amastigotes virulence. Variations in the concentration of some essential metabolites in amino sugars and nucleotide sugars metabolism, starch and sucrose metabolism, cyanoamino acid, and galactose metabolism pathways might be considered valid drug targets. Nevertheless, additional investigations need to elucidate the biochemical and molecular mechanisms of action of xanthatin.
Conflict of interests
The authors have no conflict of interest to declare.