Introduction
Non-steroidal anti-inflammatory drugs (NSAIDS) are commonly used for treatment of pain and many inflammatory disorders (1). Because of the side effects they produce, like ulcer and renal disorder, many people rely on the use of herbal medicine as a source of bioactive metabolites with less side effect (2, 3).
Genus Hydrocotyle is a genus of creeping perennial herbs of wet places, belongs to the Araliaceae family, distributed throughout the tropical and subtropical regions (4). Lin et al., reported the use of Hydrocotyle species in Taiwan folk medicine in treatment of many inflammatory diseases, dysentery, zoster, and menstrual pain (5). The main secondary metabolites detected in most Hydrocotyle species were flavonoid glycosides and triterpenoids, to which several biological activites of Hydrocotyle species could be attributed (6-10).
Hydrocotyle umbellata L. is a creeper herbaceous plant, widely distributed in the Americas (11), characterized by long cylindrical petiole attached to the center of peltate-shaped leaves. The flowers are small, white-coloured, and clustered on long peduncle that exceeds the length of the leaf petiole (12, 13). It has a great interest in folk medicine because of its anti-inflammatory, anxiolytic, and memory stimulant activities (5, 14). Florentino et al., and Rocha et al., reported the analgesic, anti-anflammatory and anxiolytic activities of the ethanolic extract of H. umbellata L. underground parts (11, 15). Due to the interesting medicinal value of H.umbellata L. and lack of reports concerning the biological and chemical investigation of H.umbellata L. aerial parts, this study was designed to evaluate the anti-inflammatory activity of the DEE of H.umbellata L. aerial parts, followed by further chemical analysis to isolate and characterize its major phytoconstituents that might be correlated to its biological activity.
Experimental
Plant material
The Samples of H. umbellata L. aerial parts (leaves and flowers) were collected in May 2014 from El-Orman Botanical Garden, Giza, Egypt and were cultivated in Experimental Station for Aromatic and Medicinal plants, Faculty of Pharmacy, Cairo University, Giza, Egypt. The plant identity was authenticated by Eng. Threase Labib, consultant in Orman Garden and National Gene Bank, Ministry of Agriculture and confirmed by Dr. Mohammed El-Gebaly, senior taxonomist at National Research Center. A voucher specimen (No. 17-8-2016) was kept at the Herbarium of Pharmacognosy Department, Faculty of Pharmacy, Cairo University.
Preparation of DEE and different extractives
The air-dried powdered aerial parts (2.3 kg) were defatted using n-hexane, then extracted with 70% ethanol till exhaustion. The combined ethanolic extracts were evaporated under reduced pressure to dryness yielding 164.2 g dry residue of DEE (7.1%). An aliquot of this dry residue (110 g) was suspended in water (400 mL) and successively partitioned with methylene chloride, ethyl acetate, and n-butanol saturated with water. Each fraction was evaporated under reduced pressure to dryness yielding 4.4 (4%), 11 (10%) and 18.7 (17%) g of each fraction, respectively. The fractions were kept in tightly closed glass containers and kept in a desiccator for phytochemical and bioactivity studies.
Chemicals for anti-inflammatory activity, total phenolics and total flavonoids
Inflammatory-grade carrageenan was purchased from FMC (Rockland, ME). Indomethacin was purchased from Sigma-Aldrich (Taufkirchen, Germany). IL-6 was purchased from (Boster Biological Technology Co., Inc., Valley Ave Pleasanton, CA). PGE2 was purchased from (Research and Development Systems, MN, USA). Folin-Ciocalteu obtained from Loba-Chemie (Mumbai, India). All other chemicals and standards were purchased from Sigma–Aldrich (St Louis, MO, USA).
Experimental animals
Adult male Wistar rats obtained from the animal house colony, National Research Center, Giza, Egypt, weighing (150-200 g) were used for determentaion of LD50 and anti-inflammatory study. They were housed at a temperature 23 ± 2 ºC and 55 ± 5% humidity with 12 h light/dark cycle, with free access to standard food pellets composed of vitamins mixture (1%), minerals mixture (4%), corn oil (10%), sucrose (20%), cellulose (0.2%), casein (10.5%), and starch (54.3%). Water was supplied adlibitum. The experimental protocol followed the Institutional Animal Ethical Committee of the National Research Centre.
Median lethal dose LD50
LD50 of the DEE of H. umbellata L. aerial parts was performed as per OECD-425 guidelines (16). Five Wistar albino rats of uniform weight were selected. One animal was fasted overnight with free access to drinking water. They were given 2000 mg/kg of the test extract (DEE) and observed for 24 h for mortality. The animal survived and then four additional animals were tested sequentially, so that a total of five animals were tested. All the animals were observed closely for 24 h and daily for 14 days, no mortality was observed.
In-vivo anti-inflammatory activity using carrageenan-induced rat paw oedema
It was carried out according to the carrageenan-induced rat paw oedema method (17). Four groups of adult male albino Wistar rats were used (1-4), each of 6 animals. Groups 1 and 2 received the vehicle (5% carboxymethylcellulose). Animals of group 3 were given indomethacin orally in a dose of 10 mg/kg b.wt. as a standard anti-inflammatory drug, and the remaining group was orally received the DEE in a concentration of 100 mg/kg b.wt. One hour later, group 1 received 0.05 mL of saline, whereas groups 2–4 received 0.05 mL of carrageenan (1% solution in saline) subcutaneously on the plantar surface of the right hind paw. The rats were sacrificed 3 hr after the induction of inflammation. The right hind paw volume was measured immediately after carrageenan injection by water displacement using a plethysmometer (model 7140, Ugo Basile, Comerio, Italy). The paw volume was re-measured 1, 2, and 3 h after injection of carrageenan (18).
The mean response for increase in the paw oedema after acute inflammation was calculated:
Oedema% = Weight of the right paw – Weight of the left Paw/Weight of the left paw × 100
Furthermore, the percentage of inhibition in the mean of the treated group in comparison with the control non-treated group was estimated and calculated according to the following equation:
Inhibition% = Paw edema of control - Paw edema of treated/Paw edema of control × 100
Measurment of IL-6 and PGE2 levels in the rat paw
Right hind paws were removed. A volume of 0.1 mL of saline containing 10µM indomethacin was injected to aid removal of the eicosanoid-containing fluid and to stop further production of IL-6 and PGE2. Paws were incised with a scalpel and suspended off the bottom of polypropylene tubes with Eppendorf pipette tips to facilitate drainage of the inflammatory exudates. For the purpose of the removal of the inflammatory exudates, the paws were centrifuged at 4000 rpm for 15 min at 4 °C and the supernatants were separated and assayed. IL-6 and PGE2 were quantified in the collected exudates using enzyme- linked immunosorbent assay kits. Both assays are based on the sandwich technique, in which specific antibodies to IL-6 or PGE2 were pre-coated on to 96-well plate. The specific detection antibodies were biotinylated. The test samples and biotinylated detection antibodies were added sequentially followed by washing. Avidin-biotin-Peroxidase complex was added and unbound conjugates were washed. A substrate solution is added to the wells to determine the bound enzyme activity. The colour development is stopped, and the absorbance is read at 450 nm using an ELISA microplate reader (ChroMate-4300, Palm City, FL). The quantifications of IL-6 and PGE2 were done according to the instructions of ELISA kits.
Statistical analysis
The data were expressed as the means ± SEM. The differences between groups were tested by one-way analyses of variance followed by the Tukey post hoc test. All statistical analyses were performed using Graph Pad Instat software version 3 (ISI software, Marinadel Rey, CA). The probability of p < 0.05 was considered statistically significant.
Toatal phenolics and total flavonoids
Spectrophotometric determination of total phenolic content was carried out using the Folin-Ciocalteu colourimetric method (19), while the total flavonoids were determined using AlCl3 colourimetric assay (20).
Chemicals for phytochemical investigation
Authentic reference samples used in co-chromatography were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Silica gel H 60 for vacuum liquid chromatography (VLC) was purchased from E-Merck (Darmstadt, Germany). Silica gel 60 and silica gel RP-18 for column chromatography were obtained from Fluka, Sigma-Aldrich Chemicals, Germany. Sephadex LH-20 was purchased from Pharmacia Fine Chemicals AB (Uppsala, Sweden). Precoated TLC plates and silica gel 60 F 254 was obtained from Fluka, Sigma-Aldrich Chemicals, Germany. The chromatograms were visualized under UV light (at 254 and 366 nm) before and after exposure to ammonia vapor, as well as spraying with p-anisaldehyde/sulfuric acid (21) and/or natural products/polyethylene glycol (NP/PEG) spray reagents (22). Shift reagents for UV spectroscopy according to the published procedures (23) and the chemicals used were obtained from E-Merck, Darmstadt, Germany.
Apparatus and equipmentfor phytochemical investigation
Schimadzu double beam spectrophotometer (UV-1650, Japan) was utilized for determination of UV shifts of flavonoids. NMR spectra were recorded at 400 (1H) and 100 MHz (13C) on a Bruker NMR-spectrometer, Japan. The NMR spectra were recorded in deuterated CD3OD and DMSO using TMS as an internal standard.
Phytochemical investigation of the ethyl acetate fraction
The ethyl acetate fraction (10 g) was chromatographed on a vacuum liquid column (VLC) (12.5 × 7 cm), packed with 190 g silica gel H 60. Gradient elution was performed starting with methylene chloride and gradually increasing the polarity with ethyl acetate by 10% increments till 100% ethyl acetate followed by increasing the polarity with methanol in 5% increments till 100% pure methanol. The fractions were collected (300 mL each) and monitored by TLC and the similar ones were pooled yielding five fractions designated as fractions I-V. Fraction I (eluted with 40-70% EtOAc in CH2Cl2) was purified on sephadex LH-20 column using methanol: water (50:50) v/v for elution to yield 100 mg of yellow microcrystals (C1). Fraction II (eluted with 5% methanol in EtOAc) was chromatographed on sephadex LH-20 column using methanol: water (50:50) v/v for elution to yield 50mg of a yellow microcrystals (C2). Fraction III (eluted with 10-15% methanol in EtOAc) was chromatographed on a sephadex LH-20 column using methanol: water (50:50) v/v for elution to yield 40 mg of yellow microcrystals (C3). Fraction IV (eluted with 20-25% methanol in EtOAc) was rechromatographed on a VLC silica gel RP-C18 column (2 × 20 cm) using methanol: water for elution with gradual 5% increments of methanol. Subfractions eluted with 15-20% methanol in water were pooled and rechromatographed on a sephadex LH-20 column using methanol: water (50:50) v/v for elution to yield 70 mg of a yellow microcrystals (C4). Fraction V (eluted with 30-40% methanol in EtOAc) was chromatographed on a sephadex LH-20 column using methanol: water (50:50) v/v for elution. Fractions of 5 mL were collected and similar fractions were pooled together yielding one subfraction, which was further purified on another sephadex LH-20 column using methanol: water (50:50) v/v for elution to yield 60 mg of a white amorphous powder (C5).
Results and Discussion
In-vivo anti-inflammatory activity
The inflammation induced by carrageenan is acute, non-immune and highly reproducible. The signs of inflammation including oedema, hyperalgesia and erythema develop immediately after subcutaneous injection of carrageenan due to the action of pro-inflammatory agents (24). Carrageenan stimulates the release of the pro-inflammatory cytokine interleukin 6 (IL-6) (18) and the inflammatory mediator prostaglandin E2 (PGE2), both IL-6 and PGE2 involved in initiation and amplification of the inflammation (18, 25). Therefore, inhibition of IL-6 and PGE2 production may serve to prevent or suppress a variety of inflammatory diseases such as hepatitis, cystitis, and rheumatoid arthritis (26). The inflammatory response is usually quantified by increase in paw size (oedema) that reaches its maximal level within 3 h (18). The intraplantar injection of carrageenan into adult male rats resulted in a severe inflammation and significant increase in the mean volume of the right hind paw compared to that of the untreated paws after 2 and 3 h of injection (Table 1). Pretreatment with DEE at a dose level 100 mg/kg showed a highly significant anti-inflammatory activity after 2 h and 3 h (70.73%) and (95.92%), respectively, with maximum activity after 3 h compared to standard anti-inflammatory indomethacin (Table 1). Injection of carrageenan into the rat hind paw induced a significant increase in the hind paw IL-6 and PGE2 concentrations, 3 h after injection, reaching 757% and 165% of control group, respectively. Pretreatment with DEE at a dose level 100 mg/kg caused a significant reduction in the high concentration of IL-6and PGE2 by 50% (about 75% of the activity of indomethacin) and 24.4% (slightly potent than indomethacin), (Tables 2 and 3, respectively).
Total phenolics, total flavonoids and phytochemical investigation
Available reports claimed that secondary metabolites of phenolic nature including flavonoids are responsible for several biological activities including anti-inflammatory activity, so it was a matter of interest to estimate the total phenolics and total flavonoids contents in addition to isolation and characterization of the major phenolic compounds accumulated in H. umbellata L. aerial parts (27, 28, 29, 30, and 31).
The total phenolic and total flavonoid contents were 79.282 ± 0.1 mg GAE/g and 57.999 ± 0.1 mg rutin equivalent/g dry weights, respectively.
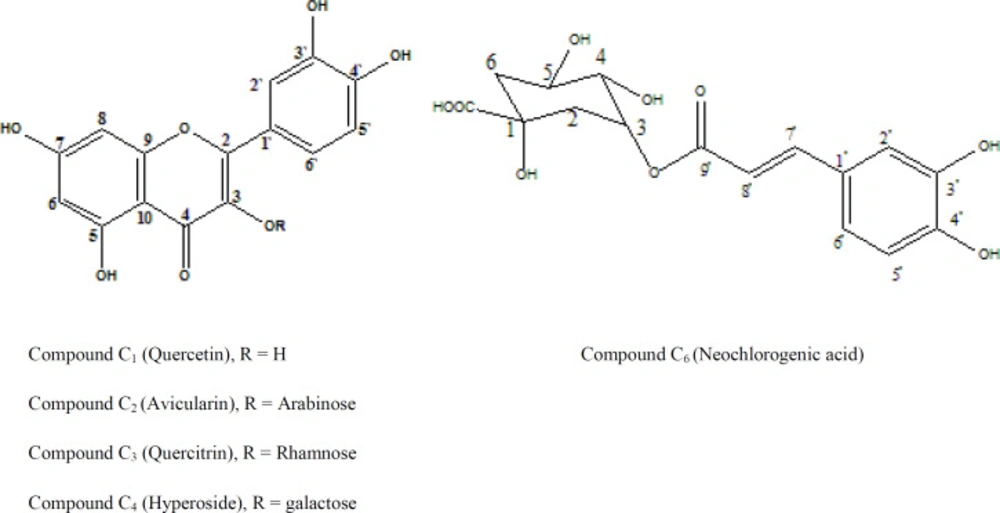
Chemical investigation of the ethyl acetate fraction resulted in isolation of four flavonoids and one phenolic acid designated as 1-5. The structures of the isolated compounds are shown in Figure 1 and their spectral data are recorded in Tables 4-7.
Compound C1 was identified as quercetin through its 1H-NMR spectrum signals, which is characteristic for quercetin nucleus and by co-chromatography with authentic sample. Compounds 2, 3, and 4 were identified as quercetin-3-O-monosaccharides. The structure of each compound was elucidated from its respective 1H and 13C-NMR spectral data. The upfield shift of their C-3 resonances in addition to their UV spectral pattern in methanol and after addition of different UV shift reagents confirmed the attachment of the sugar to C-3. Using co-chromatography with authentic samples and upon a comparison of the spectral data with the available literature compounds 3, 4, and 5 were identified as quercetin-3-O-α-arabinofuranoside (Avicularin), quercetin-3-O-α- rhamnopyranoside (Quercitrin) and quercetin-3-O-β-galactopyranoside (hyperoside), respectively (32-34). Compound C5 exihibited a sky blue colour in UV, which turns to greenish yellow colour on exposure to ammonia vapour and spraying with AlCl3. The UV spectral data of compound C5 in methanol indicated that it could be a hydroxycinnamic acid derivative (35). 1H-NMR spectrum of compound C5 showed the characteristic signals for caffeic acid. Also, the protons of a quinic acid moiety could be observed with a doublet at δ 1.73 (J = 12.9 Hz) assigned to H-6 ax, a doublet of doublet at δ 1.90 ppm (J = 13.8, 10.8 Hz) assigned to H-6 eq and a multiplet at 2.08 ppm integrated as two protons assigned to H-2 ax and H-2 eq, respectively. A broad singlet at δ 3.58 and a doublet at δ 3.62 (J = 7.08 Hz) were assigned to H-4 and H-5, respectively. The acylation of quinic acid by caffeic acid at the OH on C-3 was confirmed by the downfield shift of H-3 which appeared at δ 5.28 ppm (36). The assignment of quinic acid moiety protons was determined using 1H-1H COSY.
On the basis of the previous discussion and published data and by direct comparison with an authentic, compound C5 was identified as neochlorogenic acid (3-O-Caffoeylquinic acid) (37, 38).
| Group | Dose | 1 (h) | 2 (h) | 3 (h) | |||
|---|---|---|---|---|---|---|---|
| Paw volume (mL) | Oedema Inhibition (%) | Paw volume (mL) | Oedema Inhibition (%) | Paw volume (mL) | Oedema Inhibition (%) | ||
| Control | _ | 0.4 ± 0.01 | _ | 0.39 ± 0.01 | _ | 0.41 ± 0.01 | _ |
| Carrageenan | _ | 0.64a ± 0.03 | _ | 0.65a ± 0.02 | _ | 0.74a ± 0.02 | _ |
| Indomethacin | 10 (mg/kg) | 0.49 ± 0.01 | 23.4 | 0.41b ± 0.015 | 36.9 | 0.5b ± 0.02 | 32.4 |
| DEE | 100 (mg/kg) | 0.56 ± 0.02 | 12.5 | 0.48b ± 0.03 | 26.1 | 0.51b ± 0.02 | 31.08 |
Acute anti-inflammatory effect of the DEE of Hydrocotyle umbellata L. aerial parts
| Group | IL-6 conc. (pg/mL) (mean ± SEM) |
|---|---|
| Control | 5.6 ± 0.51 |
| Carrageenan | 48a ± 1.5 |
| Indomethacin (10 mg/kg) | 16b ± 2 |
| DEE (100 mg/kg) | 24b ± 2.1 |
Effect of oral administration of the DEE of Hydrocotyle umbellata L. aerial parts on the level of IL-6 in carrageenan-induced rat paw oedema model
| Group | PGE-2 conc. (pg/mL) (mean ± SEM) |
|---|---|
| Control | 1183 ± 128 |
| Carrageenan | 3141a ± 106 |
| Indomethacin (10 mg/kg) | 2419a,b ± 95 |
| DEE (100 mg/kg) | 2374b ± 87 |
Effect of oral administration of the DEE of Hydrocotyle umbellata L. aerial parts on the level of PGE2 in carrageenan-induced rat paw oedema model
| MeOH | Na Methoxide | AlCl3 | AlCl3/HCl | Na Acetate | Na Acetate/Boric acid | |
|---|---|---|---|---|---|---|
| C1 quercetin | 255, 301sh, 368 | 247sh, 330, 406 | 271, 305sh, 333, 458 | 267,301sh, 352sh, 429 | 268, 329sh, 390 | 261, 303sh, 387 |
| C2 avicularin | 256, 269sh, 300sh, 358 | 271, 325sh, 406 | 273, 307 sh, 335 sh, 434 | 269, 303sh, 362sh, 401 | 274, 323sh, 390 | 262, 301sh, 376 |
| C3 quercitrin | 255, 270sh, 301sh, 349 | 271, 327sh,400 | 276, 304sh,332,429 | 272, 303sh,356,400 | 271,329sh,377 | 260,292sh,368 |
| C4 hyperoside | 257, 269sh, 299sh, 362 | 272, 327sh, 410 | 275, 305sh, 335sh, 437 | 268, 300sh, 360, 405 | 274, 324sh, 390 | 262, 297sh, 376 |
| C5 neochlorogenic acid | 290, 326 | No change | ||||
UV-shifts of isolated flavonoids and phenolic acid
| Position | C1 | C2 | C3 | C4 |
|---|---|---|---|---|
| 6 | 6.08 (1H, d, J6.8 = 2 Hz) | 6.12 (1H, br.s) | 6.21 (1H, d, J6.8 = 1.96) Hz) | 6.09 (1H, br.s,) |
| 8 | 6.29 (1H, d, J8.6 = 2 Hz) | 6.34 (1H, br.s) | 6.39 (1H, d, J8.6 = 1.96 Hz) | 6.29 (1H, br.s) |
| 2' | 7.63 (1H, d, J2'.6' = 2 Hz) | 7.52 (1H, d, J2'.6' = 2.1 Hz) | 7.31 (1H, d, J2'.6' = 2 Hz) | 7.74 (1H, br.s) |
| 5' | 6.78 (1H, d, J5'.6' = 8.5 Hz) | 6.82 (1H, d, J5'.6' = 8.4 Hz) | 6.86 (1H, d, J5'.6' = 8.3 Hz) | 6.76 (1H, d, J5'.6' = 8.48 Hz) |
| 6' | 7.53 (1H, dd, J6'. 2' = 2, J6'. 5' = 8.5 Hz) | 7.49 (1H, dd J6'. 2' = 2.2 J6'. 5' = 8.2 Hz) | 7.27 (1H, dd, J6'. 2' = 2, J6'. 5' = 8.3 Hz, H-6') | 7.48 (1H, dd, J6'. 2' = 2, J6'. 5' = 8 Hz) |
| Sugar protons | 5.57 (1H, br. s, H-1''), 3.28 - 4.17 | 5.26 (1H, br.s, H-1'), 0.82 (3H, d, J = 5.92 Hz, H-6''), 3.1-4.2 | 5.05 (1H, d, J = 7.8 Hz, H-1'), 3.36 - 3.75 |
1H-NMR of the isolated flavonoids
| Position | C6 |
|---|---|
| 3 | 5.28 |
| 4 | 3.58 (br.s), |
| 5 | 3.62 (J = 7.08 Hz) |
| 2' | 6.9 (br.s), |
| 5' | 6.6 (d, J = 8 Hz) |
| 6' | 6.8 (d, J = 8 Hz) |
| 7' | δ 7.4 (d, J = 15.8 Hz) |
| 8' | 6.1 (d, J = 18.9 Hz) |
| 2ax, eq | 2.08 (2H, m) |
| 6ax | 1.73 (J = 12.9 Hz) |
| 6eq | 1.90 (J = 13.8, 10.8 Hz) |
1H-NMR of the isolated phenolic acid (C5)
| C2 | C3 | C4 | |
|---|---|---|---|
| 2 | 157.1 | 157.03 | 157.34 |
| 3 | 133.6 | 134.8 | 134.49 |
| 4 | 178 | 178.4 | 178.13 |
| 5 | 161.71 | 161.75 | 161.79 |
| 6 | 99.8 | 99.04 | 98.75 |
| 7 | 165.20 | 164.65 | 165.13 |
| 8 | 94.35 | 94.09 | 93.60 |
| 9 | 158.17 | 157.76 | 157.34 |
| 10 | 103.62 | 104.54 | 104.31 |
| 1' | 121.84 | 121.51 | 121.73 |
| 2' | 116.01 | 116.04 | 116.31 |
| 3' | 145.98 | 145.49 | 144.35 |
| 4' | 149.71 | 148.88 | 148.73 |
| 5' | 115.64 | 115.80 | 114.80 |
| 6' | 121.40 | 121.51 | 121.49 |
| Sugar protons | 108.36 (C1'') 77.20 (C2''), 82.49 (C3''), 86.45 (C4''), 61.26 (C-5'') | 102.46 (C-1''), 70.8 (C-2''), 71.04 (C-3''), 71.64 (C-4''), 70.58 (C-5''), 17.85 (C-6''). | 103.80 (C-1''), 71.81 (C-2''), 73.63 (C-3''), 68.93 (C-4''), 75.98 (C-5''), 60.62 (C-6'') |
13C-NMR of the isolated flavonoids
Conclusion
The DEE of H. umbellata L. aerial parts at a dose level 100 mg/kg showed significant anti-inflammatory activity using carrageenan-induced rat paw oedema model with significant reduction in IL-6 and PGE-2 high concentrations induced by carrageenan. Four flavonoids of quercetin nucleus and neochlorogenic acid were successfully isolated and identified from the ethyl acetate fraction of H. umbellata L. aerial parts. The isolated flavonoids and neochlorogenic acid were previously reported to exhibit anti-inflammatory activity (39, 40, 41, 42, 43 and 44). To the best of our knowledge, this is the first time of avicularin to be isolated from genus Hydrocotyle, while quercitrin and neochlorogenic acid are isolated for the first time from H. umbellata L.