Introduction
The increasing prevalence of benign breast neoplasia incidence is a public health concern in different populations (1, 2). Several lines of evidence from epidemiologic studies have given rise to the notion that environment risk factors are important in the development of breast malignancy (3).
Benign breast disease (BBD) is a well-established variable predispose women to breast cancer (BC) possibly later in life (4). The BBD consists of different histological subtypes classified as non-proliferative lesions, proliferative lesions without atypia, and atypical hyperplasia (5). Studies have shown that women with proliferative lesions of BBD are susceptible to develop malignant pathogenesis during their life time (1.5 times higher risk), whereas women with atypical hyperplastic lesions are subject to 4 times increased risk of BC (1, 3).
Among various risk factors identified in the etiology of BBD, less information exists regarding environment and diet-related factors on BBD development (6). Recently, it has been well-addressed that insulin is a tumor promoting growth factor (7). Connecting peptide or C-peptide, a marker of individual’s own insulin secretion with a long half-life, is considered to be one of the key risk factors of a later onset BC and remarked as a sensitive marker to validate the variables related to insulin levels (8). Among limited number of studies, Horner and coworkers indicated that dietary intakes of low fat and high fiber could be useful in the prevention of breast nodularity (9), suggesting that insulin metabolism could be nominated as a possible explanatory mechanism (10). To the best of our knowledge, insulin-dependent biomarkers have not been investigated in the contribution of dietary factors among patients afflicted with benign breast lesions.
Barberry or Berberis vulgaris (BV) belongs to the plant family berberidaceae (11). Its numerous biological properties have been mostly related to the high content of berberine (isoquinolinealkaloid) in BV fruit and root (12). Recent studies have shown that BV or berberine exerts therapeutic effects on some clinical manifestations of insulin resistant (IR)-dependent disorders such as diabetes (13-15). Since, the effects of BV consumption on insulin-dependent metabolic indices regarding to tumor promoting features have not been addressed thus far. Therefore, this randomized, triple-blind, and placebo-controlled clinical trial was conducted to investigate the effects of BV juice consumption on insulin-related indices among women with BBD.
Experimental
Materials and Methods
Study subjects
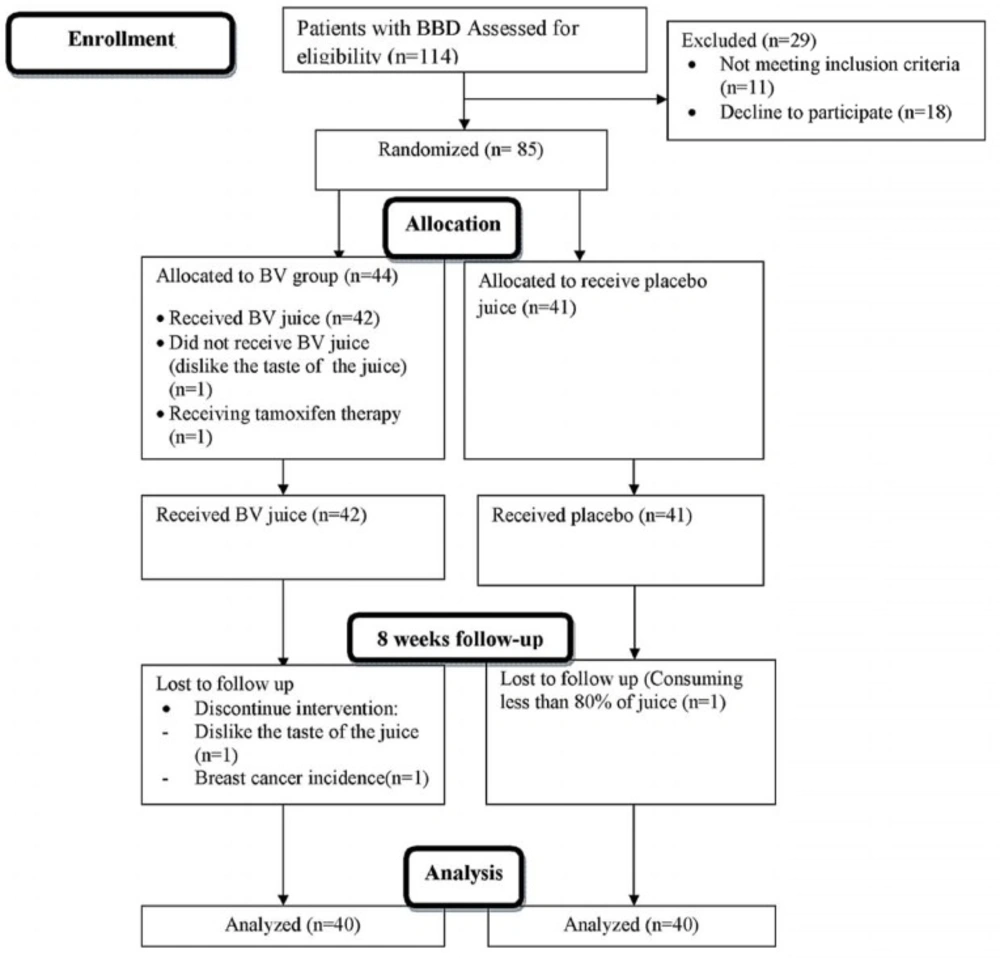
This parallel design, triple-blind, randomized and placebo controlled clinical trial was conducted on premenopausal women (19-52 years old) with benign breast tumor, whose disease was diagnosed by ultra-sonography imaging results, recruited from Nour-Nejat private hospital, Tabriz, Eastern Azerbaijan, between July 2013 and October 2014. The BBT patients did not have any surgical procedure or mastectomy prior to inclusion in the trial. Eligibility criteria included: being diagnosed with fibrocystic changes (n = 69), fibroadenoma (n = 11), written and oral informed consent, being at the interval of at least 2 years from diagnosis of BBD and no former history of malignancy in any part of the body. We excluded patients with smoking habits in previous years (ever and former smokers); pregnancy and lactating statue during the treatment; afflicted with acute and chronic illness including: cardiovascular disease, renal or liver malfunctions; other malignancies; hyperthyroidism and other hormone-related disorder (type 1 diabetes, adrenal gland disorders, polycystic ovary syndrome and hypoglycemia); gastrointestinal inflammatory illnesses (peptic ulcer, gastritis and inflammatory bowel syndrome); history of other benign lesions; any sign of bleeding and trauma reported by patients or physician; intolerance to the content of BV juice; consumption of medications like anticoagulants (such as aspirin); omega-3 (> 2000 mg/d); glucocorticoids; methotrexate; alpha-tocopherol(> 400 IU/d) supplements, epilepsy-related drugs and any medical history related to chemo-, radio-, and hormone-therapy. The sample size was calculated based on information outlined by Gu et al. (16) and estimated as 30 patients with BBT at each group, but after considering the attenuated power of analysis for stratified random sequence generation, 30% further enrolment was planned in the study protocol (Figure 1). At last, 85 women diagnosed with BBD were included and randomly assigned into two groups via stratified random allocation. All participants received written informed consent form prior to enrolment, which is completed by each participant. The study was conducted due to the revised guidelines released in the Declaration of Helsinki. The study design was approved by Ethics Committee Center at Tabriz University of Medical Sciences (Ethics no: 9233). IRCT registration number is IRCT2012110511335N2.
Study design
The selected eligible participants were asked to follow a 7 days run-in period prior to the beginning of the interventions. Run-in period was performed in order to obtain a situation to reduce the possible interaction of other dietary resources containing isoquinoline alkaloid to reach a better adherence in 8 weeks. Generally, participants was also asked not to change their common life style, particularly their usual (i.e., habitual) diet. At first interview of run-in period, dietitian instructed the participants to fill 3 days collection of 24-h dietary record (two weekdays and one on a nearest weekend), by describing portion size of food servings through the food model guideline. A dietitian carried out questioning through a face-to-face interview. The eligible participants were randomly assigned to either intervention or placebo group. Randomization was applied using a list taken out from computer-generated randomization software.
All subjects were randomized to receive 8-weeks treatment with 480 mL doses of either BV juice or placebo daily both in lunch and dinner meals (a glass in each meal equal to 240 mL). Intervention for the duration of 8 weeks was planned for this trial. All tetra pack BV juice (240 mL) was prepared by Takdaneh Agro-Industrial Company (Takdaneh, Co. Ltd., Marand, East-Azerbayjan, Iran). Each serving of BV juice contained carbohydrates: 55 gr/500 mL; protein: 600 mg/500 mL; fat: 10 mg/500mL; calcium: 50 mg/500 mL; sodium: 100 mg/500 ML and vitamin C: 80 mg/500 mL.
| Characteristics | Placebo (n = 40) | Intervention (n = 40) | P-value* | ||
|---|---|---|---|---|---|
| Mean ± S.D. | Median | Mean ± S.D. | Median | ||
| Demographic data | |||||
| Age at diagnosis (years) | 38.45 ± 6.9 | 40.0 | 36.17 ± 7.6 | 40.0 | 0.16 |
| Age at firs delivery (years) | 21.4 ± 3.9 | 20.5 | 21.0 ± 3.8 | 19.5 | 0.66 |
| Age at first menses (years) | 13.4 ± 1.6 | 13.0 | 13.0 ± 1.5 | 13.0 | 0.75 |
| Dietary intake data | |||||
| Total calorie intake (kcal/day) | 10.26 ± 4.07 | 9.07 | 11.99 ± 6.19 | 10.68 | 0.49 |
| Protein intake (g/day) | 52.49 ± 19.20 | 47.24 | 56.41 ± 22.49 | 51.49 | 0.41 |
| Carbohydrate intake (g/day) | 1359 ± 457 | 1298 | 1407 ± 483 | 1272 | 0.21 |
| Fat intake (g/day) | 5.10 ± 3.05 | 4.60 | 4.28 ± 1.93 | 4.20 | 0.25 |
| Saturated fatty acids(g/day) | 32.89 ± 12.20 | 32.49 | 37.15 ± 13.96 | 35.05 | 0.04 |
| Mono saturated fatty acids(g/day) | 9.61 ± 4.19 | 9.18 | 10.65 ± 5.09 | 9.70 | 0.16 |
| Poly saturated fatty acids | 10.47 ± 9.52 | 10.24 | 11.20 ± 10.34 | 10.36 | 0.25 |
| Dietary fiber (g/day) | 0.83 ± 1.16 | 0.76 | 0.69 ± 0.91 | 0.41 | 0.13 |
| Soluble fiber (g/day) | 113 ± 182 | 76 | 120 ± 99 | 84 | 0.64 |
| Insoluble fiber (g/day) | 13.31 ± 6.73 | 11.74 | 14.93 ± 5.66 | 13.48 | 0.61 |
Demographic and dietary characteristics of BBD patients in placebo and intervention groups at the baseline of study
| Characteristics | Intervention(n = 40) | Placebo(n = 40) | p-value* |
|---|---|---|---|
| Histopathological characteristics | |||
| Fibrocystic | 34(85.0) ** | 35(87.5) | < 0.001 |
| Fibroadenoma | 6(15.0) | 5(12.5) | |
| Others | |||
| Family history of BC | |||
| No | 35(87.5) | 30(75.0) | < 0.001 |
| Positive | 5(12.5) | 10(25.0) | |
| Family history of BBD | |||
| No | 40(100.0) | 39(97.5) | < 0.001 |
| Positive | 0 | 1(2.5) | |
| Smoker | |||
| Never | 40(100) | 40(100) | N/A |
| Ever | 0 | 0 | |
| Current | 0 | 0 | |
| Multivitamin use | |||
| No | 38(95.0) | 35(87.5) | < 0.001 |
| Yes | 2(5.0) | 5(12.5) | |
| Duration of multivitamin use (months) | |||
| ≤2 | 1(50.0) | 3(60.0) | 0.680 |
| >2 | 1(50.0) | 2(40.0) | |
| Vitamin E capsule use | |||
| No | 22(55) | 25(62.5) | 0.11 |
| Yes | 18(45) | 15(37.5) | |
| Omega3 capsule use | |||
| No | 33(82.5) | 28(70) | < 0.001 |
| Yes | 7(17.5) | 12(30) | |
Characteristics of histopathological, familial and multivitamin use status among BBD patients in placebo (n = 40) and intervention (n = 40) groups at the baseline of study
| Variable | Baseline (n = 40) | 8-weeks follow-up (n = 40) | Absolute treatment effect | Relative treatment effect | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | S.D. | P | n | Mean | S.D. | P | Mean | 95%CI | Pc | ||
| Insulin (U/mL) | ||||||||||||
| Control | 39 | 0.38 | 0.33 | N/A | 39 | 0.50* | 0.17 | N/A | N/A | N/A | N/A | 1.00 |
| BV | 35 | 0.49 | 0.43 | 0.09 | 35 | 0.51* | 0.22 | 0.72 | 0.10 | (-0.08-0.28) | 0.26 | 0.81 |
| C-peptide (ng/mL) | ||||||||||||
| Control | 40 | 2.27 | 1.06 | N/A | 40 | 2.97* | 1.13 | N/A | N/A | N/A | N/A | 1.00 |
| BV | 40 | 2.56 | 1.12 | 0.23 | 40 | 3.42* | 1.44 | 0.16 | -0.15 | (-0.62-0.32) | 0.13 | 0.92 |
| FBS (mg/dL) d | ||||||||||||
| Control | 40 | 92.22 | 14.73 | N/A | 40 | 87.85 | 12.14 | N/A | N/A | N/A | N/A | 1.00 |
| BV | 40 | 89.25 | 9.33 | 0.28 | 40 | 91.00 | 10.85 | 0.22 | -6.12 | (-12.7-0.50) | 0.97 | 1.06 |
| HOMA-IRe | ||||||||||||
| Control | 39 | 0.09 | 0.09 | N/A | 40 | 0.11 | 0.04 | N/A | N/A | N/A | N/A | 1.00 |
| BV | 39 | 0.10 | 0.09 | 0.34 | 37 | 0.11 | 0.06 | 0.19 | 0.01 | (-0.03-0.06) | 0.44 | 0.84 |
| HOMB f | ||||||||||||
| Control | 39 | 13.30 | 49.87 | N/A | 39 | 9.69* | 6.54 | N/A | N/A | N/A | N/A | 1.00 |
| BV | 36 | 10.26 | 15.86 | 0.05 | 36 | 9.80* | 9.49 | 0.05 | 375 | (21-730) | 0.86 | 1.44 |
| QUIKI g | ||||||||||||
| Control | 39 | 0.72 | 0.15 | N/A | 40 | 0.62* | 0.06 | N/A | N/A | N/A | N/A | 1.00 |
| BV | 38 | 0.67 | 0.13 | 0.15 | 37 | 0.62* | 0.07 | 0.94 | -0.04 | (-0.11-0.02) | 0.29 | 0.94 |
| G:I ratio h | ||||||||||||
| Control | 39 | 345.08 | 219 | N/A | 39 | 186.01* | 49.14 | N/A | N/A | N/A | N/A | 1.00 |
| BV | 36 | 271 | 194 | 0.11 | 36 | 185.23* | 79.07 | 0.92 | 0.15 | (-0.05-0.35) | 0.28 | 0.78 |
Serum levels of glycaemic and insulinemic biomarkers at baseline compartment of study and 8 weeks after the intervention in women with BBT who received BV supplementation (BV group) versus placebo juice consumers (control).
| Variable | Placebo group | BV group | P-value b |
|---|---|---|---|
| Insulin (U/mL) | |||
| Model 1c | 0.54 ± 0.02a | 0.55 ± 0.02 | 0.783 |
| Model 2 d | 0.54 ± 0.02 | 0.55 ± 0.2 | 0.669 |
| C-peptide (ng/mL) | |||
| Model 1 | 2.97 ± 0.07 | 3.33 ± 0.12 | 0.029 |
| Model 2 | 2.96 ± 0.07 | 3.35 ± 0.13 | 0.018 |
| FBS (mg/dL) | |||
| Model 1 | 87.64 ± 0.40 | 94.70 ± 1.24 | < 0.001 |
| Model 2 | 87.70 ± 0.41 | 94.20 ± 1.44 | < 0.001 |
| HOMA-IR e | |||
| Model 1 | 0.13 ± 0.01 | 0.14 ± 0.01 | 0.183 |
| Model 2 | 0.13 ± 0.01 | 0.13 ± 0.01 | 0.633 |
| HOMB f | |||
| Model 1 | 666 ± 78 | 973 ± 147 | 0.114 |
| Model 2 | 664 ± 76 | 977 ± 142 | 0.048 |
| QUIKI g | |||
| Model 1 | 0.66 ± 0.01 | 0.65 ± 0.01 | 0.447 |
| Model 2 | 0.66 ± 0.01 | 0.65 ± 0.01 | 0.359 |
| G:I ratio h | |||
| Model 1 | 0.62 ± 0.02 | 0.63 ± 0.02 | 0.697 |
| Model 2 | 0.62 ± 0.01 | 0.63 ± 0.01 | 0.252 |
Changes of dependent variables (plasma levels of glycemic and insulinemic biomarkers) with BV supplementation in women with BBD
Spectrophotometric method measured absorbance of berberine at 418 nm in BV juice (17). After measuring five different samples of BV juices, the average berberine content was determined 2.03 ± 0.03 μM/mL. The company declares no addition of any preservatives in BV juice tetra-pack.
Weekly checklist of BV consumption was handed in to the eligible patients for assessing the probable intolerance to interventions, detailing undefined events, doubt or accidental consumption, and other medical events. If weighted amount of remaining juice in bottle was more than 20% of total amount of juice, the subject was considered to be none respondent to the intervention. In addition, bottles were collected in every two weeks. During the intervention, data on 3-day 24 h dietary and physical activity records were collected once in every two weeks. Physical examinations at these visits were done by physician in order to determine any clinical changes during the study, including malignancy, diabetes, hepatic complications and pregnancy after enrolment and during the interventions, which were considered as exclusion criteria. Intervention follow up continued until December 2014.
Participants in placebo group consumed 480 mL of placebo juice had a content which was normalized to become identical in calorie, vitamin content, taste, size and color to the BV juice. To meet the allocation concealment criteria, white opaque plastic was used to cover juice pack similarly. Juice packs were provided in every two weeks (18). Subjects were asked to store juices in refrigerator at 4ºC. Sequence generation and allocation concealment were listed and marked by designer of study and implemented by clinic personnel who were unaware of allocation at the time of enrolment. Participants, clinic care givers who had been responsible to hand in plastic bags and also laboratory personnel were blinded. The average of nutrients intake level for each participant obtained from 24 h dietary records at baseline were analyzed by Nutritionist IV software (version 3.5.2; 1994, N-Squared Computing, San Bruno, CA). To assess the concurrence of dietary data, a validated food frequency questionnaire with 136 food items (19) was used.
Biochemical analyses
Serum samples of 80 patients were collected by considering overnight 12 h fasting. Blood sampling process had not being performed in duration of 3rd till 5th days of menstruation (follicular phase) both at baseline and after the 8-week follow-up period. Blood were transferred centrifuged (Gallenkamp centrifuge) at 3000 × g at 20 °C for 10 minutes to separate serum supernatant. Sera samples transferred into 1.5mL microtubes and stored at -70 °C until sample analysis. Outcomes of the study consist of serum levels of C-peptide, fasting blood sugar (FBS) and insulin. They were assessed using a specific enzyme linked immunosorbent assay (ELISA) kits for fasting blood sugar (FBS) (Pars-Azmoon, Cat. No: 5825; Tehran, Iran), insulin by Monobind (Cat No: 5825-300; California, USA), and C-peptide using Monobind kit (Cat No: 2725-300; California, USA). The internal coefficient variations of biomarkers were reported about 92%. The measurements were carried out following instruction provided by manufacturer. The HOMA related indices were calculated using the HOMA-IR= [FBS (mg/dL) × insulin (IU)/405] and HOMA-B= [(360 × insulin (IU)]/ [(FBS (mg/dL)-63] × 100. The quantitative insulin sensitivity check index (QUICKI) was estimated using 1/[(logarithm of insulin+ logarithm of FBS)]. To attenuate systematic errors for each biomarker, measures were done at the same time in a laboratory run and random order. A numeric label code was used instead of each patient’s name, to attain blinding at laboratory analysis.
Statistical analysis
Statistical analysis was carried out using SPSS software package (version 13.0; SPSS Inc.). Paired samples t-test was used to compare mean values of a variable from baseline to the endpoint of study within each group. Independent sample t-test was used to compare a variable between BV and placebo groups at certain time point. The mean change in the variable from baseline to the 8 weeks of follow-up between the two arms of intervention was calculated by absolute treatment effect and tested by a repeated-measures linear mixed model. The relative effect is a term used to define probability of an outcome in one treatment group relative to that in the placebo group and likewise. The odds ratio (OR) can interpret the proportional change in the treatment group relative to placebo group. Dependent variable was adjusted for baseline data and estimated through a weighted least squares (WLS) regression model. Subsequently, linear mixed model was used to consider covariates including frequency of lactation (n), and body mass index (BMI, kg/m2) at baseline. P values less than 0.05 has been reported as statistically significant.
Results
Baseline characteristics
The excluded subjects during the enrollment or during the 8-week follow-up are depicted in the flow chart diagram (Figure 1). None of the study subjects were in the follicular phase of their menstrual cycle, and all were in pre-menopausal status. The average rate of being respondent to interventions was 94.3% in case of using BV juice and 90.7% in placebo group. The BV and its placebo juice did not lead to any adverse effects in patients till the trial was completed. The baseline characteristics of participants were not significantly different between the intervention and placebo groups.
The habitual dietary components and lifestyle-related factors of all subjects did not differ significantly at the length of intervention (Table 1). Among study subjects, pathological classification of benign disease identified to be 85.0% with fibrocystic and 15.0% with fibroadenoma in the BV group. Similarly, 87.5% of participants in placebo group had fibrocystic tumor significantly different from the relative frequency of those subjects with fibroadenoma (12.5%, P < 0.001) (Table 2).
The primary outcomes
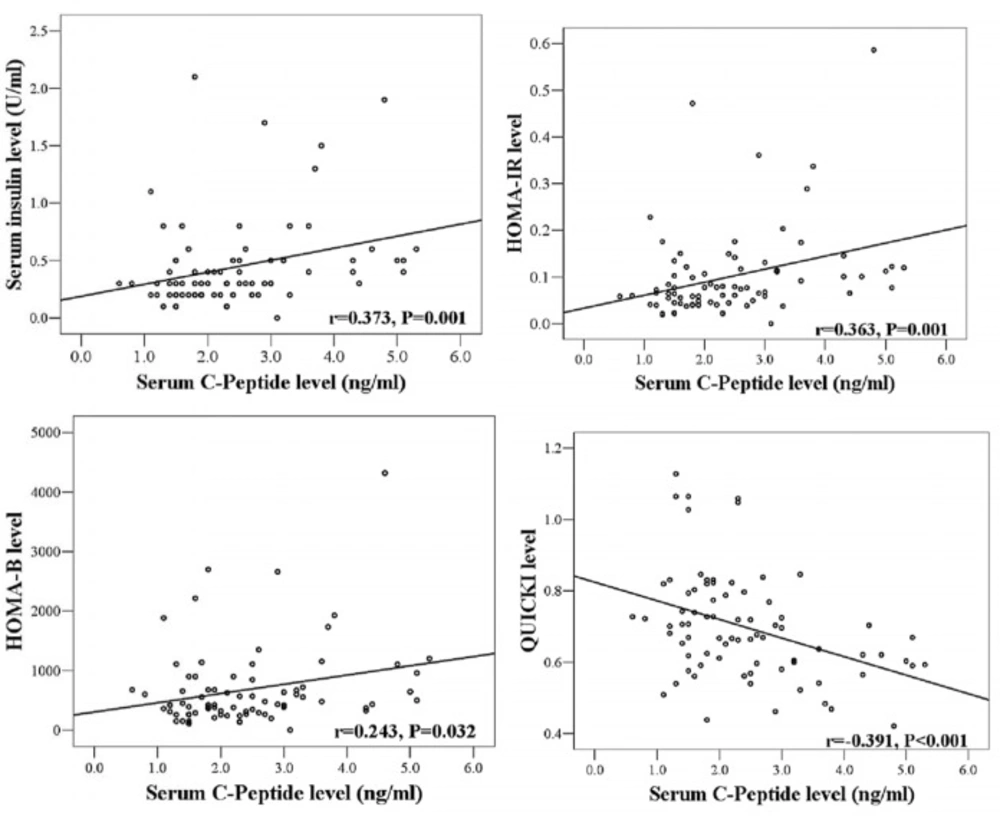
Serum levels of C-peptide correlated significantly with serum insulin levels (r = 0.37, P < 0.01) and HOMA-IR values (r = 0.36, P < 0.01). C-peptide was inversely correlated with the variable of QUICKI (r = - 0.39, P < 0.001) (Figure 2).
Although comparing the results of the 8-weeks intervention with baseline (Table 3) showed significant increases in the average levels of insulin at both groups, insulin decreased by 19% after 8 weeks intervention in BV group relative to placebo [relative treatment effect = 0.81, (P = 0.26)]. There was also a significant increase in C-peptide levels both in the placebo, and BV groups (Table 3). Serum C-peptide decreased by 8% after treatment with BV relative to placebo, whereas these changes were not statistically significant (Table 3). HOMA-IR showed 16% decrease in BV group relative to the placebo, but it was not statistically significant (Table 3). Although there were significant decreases of HOMA-B levels in both groups (P < 0.001), HOMA-B increased by 44% relative to placebo after 8 weeks treatment of the BV group (Table 3). Mean changes of QUICKI level was higher in placebo group while compared to BV group. Glucose to insulin (G: I) ratio decreased 22% in the BV group, which is comparable to the relative change observed in placebo over the 8 weeks (Table 3). Table 4 showed values adjusted for baseline variable in model 1 and subsequently further controls for potently related covariates (i.e., the frequency of lactation (n), and BMI at baseline) undertook in model 2. Results indicated that there was no significant increase in insulin level in both models (Table 4). The mean levels of C-peptide or FBS after performing adjustments for corresponding baseline values, increased significantly (P < 0.05). HOMA-IR level increased in model 1, but it did not reach the statically significant level (0.13 ± 0.01 to 0.14 ± 0.01, P = 0.18). The model 2 analysis showed a remarkable increase in HOMA-B level in post-adjustment model controlled for the frequency of lactation (n), and BMI at baseline through WLS model (Table 4).
Discussion
There is growing body of evidence showing that obesity in association with IR seems to be a causative factor for developing BC pathogenesis (4). Our findings over the present randomized controlled trial could support the assumption that BV juice might reduce HOMA-IR and G:I ratio, and in the meantime, enhance the level of HOMA-B.
The advantage of using berberine in relation to enhance insulin sensitivity in terms of HOMA-B resulted in the study of Lu and coworkers (20). It was also supported by our finding showed that BV intervention increased HOMA-B by 44% relative to placebo in WLS model.
However, to the best of our knowledge thus far, this is the first study investigated the effect of BV on insulin-related indices in benign breast disease. A clinical trial on T2DM patients for 3 months performed by Gu and colleagues showed that the daily supplementation of berberine can lead to a significant decrease in fasting and post-load glucose levels as well as glycated hemoglobin (HbA1c) (16). Consistent to our findings in BBD patients, Zhang et al. (21) reported that daily supplementation of berberine for 3 months caused significant reductions in fasting and post loaded plasma levels of glucose in T2DM patients with dyslipidemia. Affuso et al. (22) also suggested that the supplementation of a nutraceutical combination consisted of berberine, red yeast rice, and policosanols during 4 weeks might cause decrease in HOMA-IR as an indicator of insulin resistance, while significant increase was obtained in QUICKI of hypercholesterolemic patients. Diminution of G:I ratio in our analysis is in agreement with findings from randomized trial conducted in T2DM patients showed that the BV extract for 3 months could improve IR (23).
The results of estimated relative treatment effect represented that BV juice caused apparently reducing effects on insulin and C-peptide levels consistent with the findings reported by Affuso et al. (22). In contrast, post-adjusted models showed significant increase in C-peptide levels in response to BV treatment, which is consistent with Yin et al. (24) findings attributed to remarkable increase in both fasting and postprandial C-peptide levels in patients treated with combinative effects of berberine and insulin therapy (T2DM patients). However, our findings based on the estimated relative treatment effect supported present hypothesis and suggested that BV supplementation could ameliorate HOMA-IR as an IR determinant. This is in accordance with the interventional evidence postulated for T2DM (15, 24). In line with our findings, Di-Pierro et al. (15), showed that a herbal extract contained Berberisaristata can lessen the mean level of HOMA-IR in T2DM patients. Taken together, BV supplementation seems to cause general controlling effects on glycemia in keeping with improvement on insulin secretion profile to possibly overcome the physiologic condition describing less insulin responsiveness. Additionally, Lu and coworkers found out that berberine treatment in diabetic rats could increase insulin level and preserving β-cell number in pancreas (20). Accordingly, to support the provided insights on the plausible benefits of BV fruit, the raised relative treatment effects observed in HOMA-B as an indicator of β-cell functionality suggested BV as an effective herb on enhancing HOMA-B in patients with BBD (20, 25).
The current study was associated with some limitations. Most importantly, the duration of consumption of BV juice was short and remains unclear whether the longer period of time may accompany with stronger evidence on insulin related indices. Also, another problem with this approach is that there was no similar controlled clinical trials published about BBD to be compared in results and almost all the clinical trials have tended to focus on the effects of BV on diabetic models.
Conclusion
This study suggested that BV juice caused regulatory roles on HOMA-IR and enhanced HOMA-B with the possible metabolic controlling effects on the growth promoting outcome of insulin. Taken together, our findings suggest that regular BV juice consumption might be effective on controlling insulin-related indices in benign breast disease.