Introduction
Cardiovascular diseases are the leading causes of death and disability in most countries of the world. Coronary heart disease (CHD) is the most common type of cardiovascular disease. (1) In the United States, CHD annually results in 502,000 deaths, and an economic burden of $133 billion. (2). Percutaneous coronary intervention (PCI) is a common non-surgical procedure used to open narrow or blocked coronary (heart) arteries and restore blood flow to the heart muscle. Major Adverse Cardiac Events (MACE) including stent-thrombosis, recurrent MI, death and need for urgent revascularization can occur during and after PCI. Activation and aggregation of platelets has an important role in the pathogenesis of these complications so, FDA currently recommends clopidogrel and aspirin for reduction of these events (3).
Clopidogrel is a pro-drug that is predominantly catalyzed by cytochrome P450 (CYP) 2C19, 2C9, and 3A4 enzymes, (4, 5) and inhibits platelet activation through a selective and irreversible blockade of the adenosine diphosphate (ADP) P2Y12 receptors (6). Inter-individual variability in platelet response to clopidogrel, ranging from 5 to 44% (7-12) with clinical relevance (5, 8, 13-17) is well recognized. So, monitoring of platelet function to identify non-responders, which are in increased risk of MACE, could be useful.
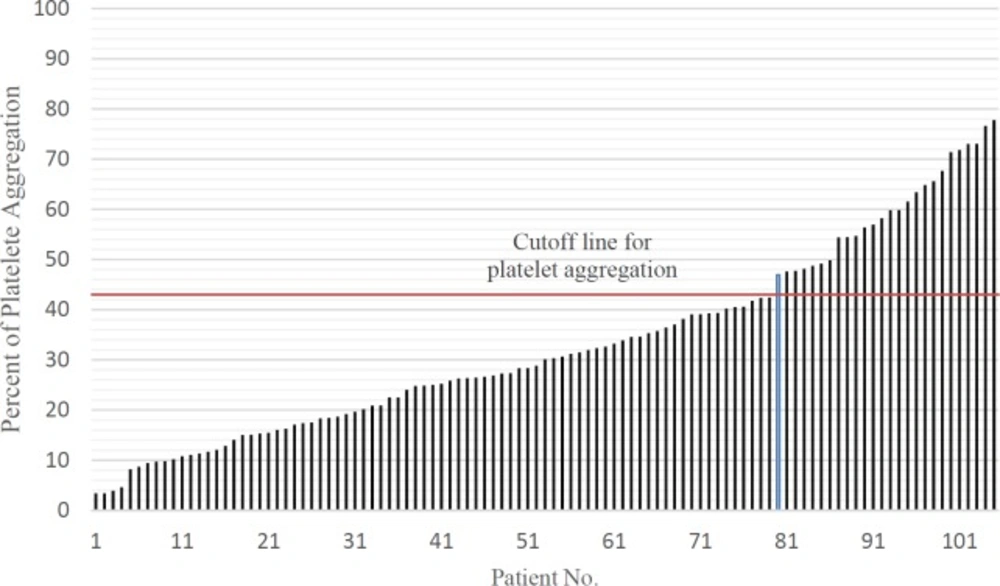
Adenosine diphosphate (ADP)-induced light transmittance aggregometry (LTA) is the gold standard test for evaluating platelet reactivity in patients receiving clopidogrel.(18) In this method platelet aggregation more than 43% induced by 5 mM ADP have been defined as clopidogrel non-responder (19). Data about clopidogrel resistance in Iranian population is scanty. The primary objective of this study was to investigate the prevalence of clopidogrel–resistance and secondary objective was evaluation of contributing risk factors to clopidogrel among Iranian patients. Also we evaluated patients MACE criteria 1 month and up to 3 years after PCI.
Experimental
The population in this study was comprised of patients undergoing angioplasty with a drug eluting stent (DES) placement that were admitted to the coronary angiography, CCU, post-CCU and Cs-ICU of Imam Hossein Medical Center, affiliated to Shahid Beheshti Medical University, Tehran, Iran. All patients were given a written informed consent before entering the study, and the study was performed in accordance with the declaration of Helsinki and was approved by the Medical Ethics Review Board of Shahid Beheshti University of Medical Sciences. Patient were included in the study if they were subjected to angioplasty procedure with DES and consuming standard dosage of aspirin (75-325mg/day). The exclusion criteria were age under 18, history of clopidogrel usage in last 1 month, acute infarction in last 18 h platelet count < 100000/mm3, hematocrit < 25%, creatinine > 4mg/dL, usage of glycoprotein IIb/IIIa before procedure, history of alcohol consumption, previous PCI, contraindications for anticoagulant therapy, severe disease with life expectancy under 1 year, cancer and dialysis.
Blood sampling from patients was performed from June 2011 to July 2013. For all patients entering the study, a questionnaire including demographic information including age, sex, past medical history (such as MI, HTN, HLP, DM, history of PCI, IHD), drug and habitual (such as smoking, addiction) history was completed and their correlation to clopidogrel resistance were evaluated with relative statistical tests.
All patients received a loading dose of 600 mg clopidogrel (Plavix®, Sanofi, France) before the angioplasty and a daily dose of 75 mg afterward. Platelet aggregation test was performed for all patients 24 h after taking the loading dose of clopidogrel using light transmission aggregometry (LTA) method using a 4-channel Labtec APACT 4004 aggregometry and platelet aggregation equal and greater than 43% considered as clopidogrel non-responders.
Statistical Analysis
Data were analyzed by SSPS version 17.0 (SPSS, Inc., Chicago, IL, USA) with related tests. The results are expressed as a mean ± standard deviation (SD), and/or range. p values less than 0.05 were considered statistically significant.
Results
One hundred and five patients with mean age of 60.30 ± 12.2 entered the study. Sixty-four patients were female and the hypertension was medical history most common (66%) in subjects. Demographic findings, past medical and drug history of patients are summarized in Table 1. In our study, these factors including history of PCI, addiction, MI, IHD, HLP, smoking, DM, HTN do not have significant association with clopidogrel resistance.
The mean platelet aggregation in study population was 32.94% ± 18.83 (ranged from 3.41% to 77.79%). Twenty-six patients (24.76%) had platelet aggregation above 43% which were categorized as non-responder to clopidogrel. the data are shown in Figure 1.
| Parameter | Total | Clopidogrel responder | Clopidogrel | p-value |
|---|---|---|---|---|
| Age (mean ± SD) | 60.30±12.2 | 59.15 ± 11.4 | 63.8 ± 12.7 | |
| Female Sex; No. (%) | 64 (61%) | 50 (63.29) | 14(53.84) | 0.23 |
| Smoker (No) | 24.76 | 29.11 | 11.54 | 0.07 |
| Addict (No) | 3.81 | 3.80 | 3.70 | 0.99 |
| PMH; No. (%) | ||||
| DM | 21(20.95) | 16(20.25) | 5(23.08) | 0.75 |
| HTN | 69(65.71) | 52(65.82) | 17(65.38) | 0.96 |
| IHD | 11(10.48) | 9(11.4) | 7.69 | 0.59 |
| HLP | 15(14.28) | 11(13.92) | 4(15.38) | 0.85 |
| MI | 7(6.66) | 5(6.32) | 2(7.7) | 0.8 |
| PCI | 5(4.76) | 5(6.32) | 0(0) | 0.18 |
| Past Drug history | ||||
| PPIs | ||||
| Pantoprazole | 11(10.5) | 11(13.92) | 0(0) | 0.04 |
| Omeprazole | 5(4.76) | 1(1.26) | 4(15.38) | 0.003 |
| Atorvastatin | 64(60.95) | 51(64.55) | 13(50) | 0.18 |
| Beta-blocker | 62(59.04) | 48(60.79) | 14(53.84) | 0.53 |
Demographic findings, past medical and drug history of 105 patients entering the study
One month and 3 years after PCI, patients MACE criteria were followed by telephone call. After one month, none of the patients experienced MACE in responder and non-responder groups. Three years after PCI, the data had been collected from 65 (61.90%) individuals, 55 (69.62%) of whom were in clopidogrel responder arm and 10 (38.46%) in non-responder group. MACE criteria was positive in 4 patients, 3 (5.45%) in responder and 1 (10%) in non-responder group. There was no significant difference between two arms in MACE criteria (p = 0.28).
Also we analyzed the association of possible risk factors with resistance to clopidogrel. We did not find any significant differences between clopidogrel resistance and history of HTN, HLP, DM, PCI, IHD, and MI.
In drug history 11 (13.92%) of patients in responder group were receiving pantoprazole while none of patients in non-responder group received this agent concurrent with loading dose of clopidogrel (p = 0.04). One (1.26%) and 4 (15.38%) patients received omeprazole with clopidogrel in responder and none-responder group, respectively (p = 0.003). There was no significant difference in other medications including atorvastatin, nitrate, and beta blockers in two groups.
Discussion
The results of current study showed that the platelet function did not decreased to less than 43% in 24.76% of patients after receiving 600mg loading dose of clopidogrel. We did not detect any significant difference in MACE criteria in responder and non-responder patients in phone call follow-up 1 month and 3 years after PCI. Furthermore, we did not find any correlation between patient’s characteristics and response to clopidogrel, except treatment with omeprazole.
In a systematic review by Snoep et al. mean unadjusted prevalence of clopidogrel resistance, weighted for study size, reported to be 21 % (95% CI, 17%-25%) which is in accordance with results of our study(20). Lapantalo et al. evaluated 1608 Finnish patients and reported 20% resistance among which is line of with our study (21). In German population, Schulz et al. and Geisler el al. reported 20% and 5.8% resistance to clopidogrel respectively. (22-24). Although, the results of study by Geisler el al. differ from our reported percent, it is consistent with those of Schulz et al.
In Iranian population in 2013, our team measured platelet aggregation pre and post treatment with clopidogrel in 31 patients and divided patients into three groups. The results showed that 22% and 13% of the patients were semi-resistant and none-responder to clopidogrel, respectively (12). Also our results are in agreement with Namazi’s (2012) findings which showed 26% of clopidogrel non-responsiveness, in Iranian population (25).
In contrast to earlier findings, however, no evidence of relationship between underlying disease including DM, HTN, IHD, PCI, MI, and habitual history including addiction and smoking was detected in our study. Al-Azzam et al. evaluated the factors that contribute to clopidogrel resistance and reported that use of calcium channel blockers and low HDL have significant association with clopidogrel resistance while age, diabetes, hypertension, smoking and aspirin use did not have significant contribution to clopidogrel resistance (24). In another study, ZHANG chun-ying et al. observed significant association between clopidogrel resistance and history of smoking, diabetes, fasting plasma glucose, glycosylated hemoglobin, triglyceride, and cardiac troponin (26).
de Miguel Castro, A., et al. (2009) showed that lower response to clopidogrel were significantly associated with MACE occurrence in 1-year follow-up (27). This differs from the findings presented here maybe due to study limitations particularly missed follow-up in non-responder patients which consisted of more than 60% of the subjects. The other limitation of current study is its single center design and small sample size for detection of MACE criteria.
Conclusion
The results of this investigation show that there is no major difference between Iranian population and other studies regarding the resistance to clopidogrel. The other findings were lack of correlation between clopidogrel resistance and past medical and drug history. Also the MACE criteria was not different between responders and non-responders in the current study. Due to the study limitations, further multi center investigations with higher number of patients and accurate follow up of the patients is recommended.