Introduction
Heterocyclic compounds containing pyran, pyrimidine, and pyrazole moieties have been found to possess various biological and pharmacological properties. The fused pyran ring is a prominent structural motif found in a variety of bioactive compounds such as anticoagulant (1), anticancer (2) antimicrobial (3) antimalarial (4, 5) anti-HIV (6) and anti-fungal agents (7). Substituted pyrimidines constitute a class of biologically important compounds showing analgesic and anti-inflammatory activities (8, 9) and also act as potential kinases inhibitors (10, 11). Furthermore, pyrazole derivatives are important structural subunits with a broad range of pharmacological properties such as anticancer (12) antibacterial (13) analgesic (14) and anti-inflammatory activities (15). Pyranopyrazoles and fused pyrimidines especially pyrazolopyranopyrimidine derivatives exhibit unique potential anti-inflammatory, analgesic, antipyretic, and anti-tubercular activities (Figure 1) (16-18).
In modern organic chemistry, considerable efforts have been devoted to the design of strategies leading to structurally diverse and complex molecules (19-21). In this context, multicomponent reactions (MCRs) offer significant advantages over conventional linear-type syntheses. Such protocols allow molecules to be assembled from three or more starting materials in a one-pot process (22, 23).
Propyl sulfonic acid functionalized SBA-15 as a heterogeneous Brønsted acid with a hexagonal structure, high surface area, and large pore size exhibits efficient catalytic activity in a variety of organic reactions (24). In continuation of our previous studies on the application of nanoporous solid catalysts in organic reactions (25-28) herein, we report on the design and optimization of a convenient MCR approach for the synthesis of pyrazolopyranopyrimidine derivatives using SBA-Pr-SO3H as a nano-catalyst. Some of the synthesized compounds displayed significant antimicrobial activity against some fungi and gram positive and negative bacteria.
Experimental
Chemical compounds employed in this work were purchased from Merck Company and used with no purification. IR spectra of samples were recorded on FT-IR Bruker Tensor 27 instrument. Melting points were measured using the capillary tube method with an Electrothermal 9200 apparatus. The 1H (250 MHz) and 13C NMR (62.5 MHz) were run on a Bruker DPX using tetramethylsilane (TMS) as an internal standard (DMSO-d6 solution). Mass spectra data were obtained using the network mass selective detector (Agilent) 6890/5973. SEM analysis was undertaken on a Philips XL-30 field-emission scanning electron microscope operated at 16 kV. TEM analysis was performed on a Tecnai G2 F30 at 300 kV.
Preparation of catalyst
The nanoporous compound SBA-15 was synthesized and functionalized according to a previous report (29) and the modified SBA-Pr-SO3H was used as a catalyst in the following reaction.
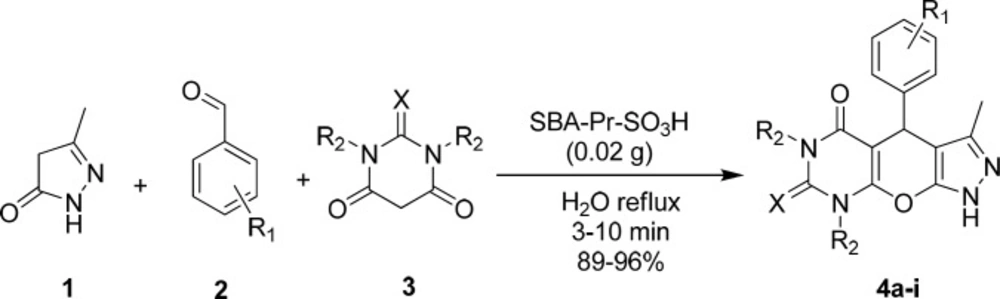
Synthesis of pyrazolopyranopyrimidines in the presence of SBA-Pr-SO3H under reflux conditions.
| Entry | Catalyst | Solvent | Condition | Time (min) | Yield (%) | Year |
|---|---|---|---|---|---|---|
| 1 | DABCO | H2O | Reflux | 20-45 | 84-99 | 2014 (30) |
| 2 | Meglumine | H2O | Stir. (r.t.) | 15-60 | 89-95 | 2014 (31) |
| 3 | SBA-Pr-SO3H | H2O | Reflux | 3-10 | 89-96 | This work |
Comparison of efficiency of various catalysts in the synthesis of pyrazolopyranopyrimidines
| Compound | B. subtilis | S. aureus | E. coli | P. aeruginosa | C. albicans |
|---|---|---|---|---|---|
| 4a | 20 | 24 | 12 | 0 | 28 |
| 4b | 18 | 22 | 12 | 0 | 20 |
| 4c | 15 | 21 | 13 | 0 | 20 |
| 4d | 14 | 19 | 12 | 0 | 20 |
| 4e | 16 | 24 | 0 | 0 | 0 |
| 4f | 21 | 24 | 12 | 0 | 26 |
| 4g | 19 | 23 | 14 | 0 | 20 |
| 4h | 21 | 25 | 16 | 0 | 24 |
| 4i | 16 | 20 | 12 | 0 | 20 |
| Chloramphenicol | 26 | 22 | 24 | 8 | - |
| Gentamicin | 28 | 20 | 20 | 18 | - |
| Nystatin | - | - | - | - | 18 |
Inhibition zone (mm) of synthesized compounds against some gram positive and gram negative bacteria and fungi, by disc diffusion method (IZ = 250 µg/disc).
| Compound | B. subtilis | S. aureus | E. coli | P. aeruginosa | C. albicans |
|---|---|---|---|---|---|
| 4a | 32 | 8 | 256 | - | 2 |
| 4b | 64 | 16 | 256 | - | 32 |
| 4c | 128 | 32 | 128 | - | 32 |
| 4d | 128 | 32 | 256 | - | 32 |
| 4e | - | - | - | - | - |
| 4f | 16 | 8 | 256 | - | 4 |
| 4g | 32 | 16 | 128 | - | 32 |
| 4h | 16 | 8 | 64 | - | 8 |
| 4i | - | - | - | - | - |
| Chloramphenicol | 4 | 8 | 4 | 256 | - |
| Gentamicin | 0.125 | 0.5 | 0.5 | 1 | - |
| Nystatin | - | - | - | - | 8 |
Minimum inhibitory concentration (µg/mL) of synthesized compounds against some gram positive and gram negative bacteria and fungi
Procedure for the synthesis of 3-methyl-5-pyrazolone
A solution containing hydrazine hydrate 80% (1.4 mmol, 0.07 g) and ethyl acetoacetate (1 mmol, 0.13 g) in ethanol (10 mL) was stirred at room temperature for 5 min. After the completion of reaction as indicated by TLC, the solution was diluted with ethanol (10 mL) and stirred in an ice bath for 30 min. The resultant solid was then filtrated, washed with cold ethanol, and recrystallized from ethanol to give pure 3-methyl-5-pyrazolone.
General procedure for the synthesis of pyrazolopyrznopyrimidines
A mixture of barbituric acid (1 mmol, 0.128 g), aromatic aldehydes (1 mmol), 3-methyl-5-pyrazolone (1 mmol, 0.098 g), and SBA-Pr-SO3H (0.02 g) was refluxed in water (4 mL) for the appropriated length of time. After completion of the reaction (monitored by TLC), the generated solid product was dissolved in hot ethanol and acetone (1:1), the heterogeneous solid catalyst was insoluble and could be removed by filtration. The pure products 4a-i were obtained after cooling of the filtrates. The catalyst was washed with diluted acid solution, water, and then acetone, dried under vacuum and reused for several times. The physical and spectral data of new compounds are given below:
3,6,8-Trimethyl-4-phenyl-6,8-dihydropyrazolo[4ꞌ,3ꞌ:5,6]pyrano-[2,3-d]pyrimidine-5,7(1H,4H)-dione (4h)
White solid, Yield: 96%, M.p. 208-210. IR (KBr) ν: 2923, 1683, 1572, 1468, 1386, 1323, 1276, 1235, 1142, 822, 698 cm-1. 1H NMR (250 MHz, DMSO-d6) δ: 2.23 (s, 3H, CH3), 3.11 (s, 6H, CH3), 5.54 (s, 1H, CH), 7.00-7.19 (m, 5H, ArH), 13.5 (br s, 1H, NH) ppm. 13C NMR (62.5 MHz, DMSO-d6) δ: 10.4, 28.2, 32.5, 91.6, 106.3, 125.7, 127.1, 128.3, 142.8, 144.2, 152.1, 159.5, 163.7 ppm. Mass m/z (%): 324 (14), 243 (100), 186 (73), 156 (58), 42 (91). Anal. Calcd for C17H16N4O3: C, 62.95; H, 4.97; N, 17.27. Found: C, 62.88; H, 5.05; N, 17.21.
3-Methyl-7-thioxo-4-(p-tolyl)-4,6,7,8-tetrehydropyrazolo[4ꞌ,3ꞌ:5,6]pyrano[2,3-d]pyrimidin-5(1H)-one (4i)
White solid, Yield: 91%, M.p. 219 °C. IR (KBr) ν: 3421, 2922, 1623, 1533, 1508, 1224, 649, 511 cm-1. 1H NMR (250 MHz, DMSO-d6) δ: 2.18 (s, 3H, CH3), 2.21 (s, 3H, CH3), 5.37 (s, 1H, CH), 6.88 (d, J = 7.7 Hz, 2H, ArH), 6.98 (d, J = 7.7 Hz, 2H, ArH), 11.49 (s, 2H, NH), 13.48 (br s, 1H, NH) ppm. 13C NMR (62.5 MHz, DMSO-d6) δ: 10.4, 20.9, 30.6, 96.6, 106.0, 126.9, 128.9, 134.8, 139.0, 139.1, 144.2, 159.6, 159.7, 163.9, 173.4 ppm. Mass m/z (%): 326 (2.5), 281 (2.5), 246 (20), 199 (100), 185 (71), 115 (59). Anal. Calcd for C16H14N4O2S: C, 58.88; H, 4.32: N, 17.17. Found: C, 58.79; H, 4.23: N, 17.25.
General procedure for in-vitro antibacterial evaluation of compounds 4a-i
The biological activities of compounds 4a-i were screened in-vitro using the disc diffusion method (IZ) and subsequently the minimum inhibitory concentration method (MIC). The microorganisms used were Pseudomonas aeruginosa (ATCC 85327) and Escherichia coli (ATCC 25922) as gram-negative bacteria, Staphylococcus aureus (ATCC 25923) and Bacillus subtilis (ATCC 465) as gram-positive bacteria, and Candida albicans (ATCC 10231) as the fungus. All obtained compounds were dissolved in DMSO (100 µg/mL) and 25 µL was loaded onto 6-mm paper discs. One hundred microliters of 109 cell/mL suspension of the microorganisms was spread on sterile Mueller–Hinton agar plates, and the discs were placed on the surface of culture plates. The MIC of the synthesized compounds which showed antibiotic activity in disc diffusion tests was also determined by microdilution method and activities of each compound were compared with chloramphenicol, gentamicin, and nystatin as references.
Results and Discussion
Herein, we report a green and highly efficient method for the synthesis of pyrazolopyranopyrimidine derivatives through the one-pot condensation of 3-methyl-5-pyrazolone 1 (1 mmol), aromatic aldehydes 2 (1 mmol), barbituric acid 3 (1 mmol), and SBA-Pr-SO3H as the heterogeneous catalyst in water (Scheme 1). 3-Methyl-5-pyrazolone 1 was synthesized in the way that was mentioned in experimental section. To optimize the reaction conditions, barbituric acid, 4-chlorobenzaldehyde, and 3-methyl-5-pyrazolone in a molar ratio of (1:1:1) were selected as a model reaction. Comparing the reaction times and yields of products under different conditions such as using water as the solvent (under reflux and room temperature) and also solvent free condition, the best result (92% yield) was obtained under reflux conditions in water after 10 min in the presence of SBA-Pr-SO3H (0.02 g). As shown in Table 1, the presence of the catalyst was found to give a higher yield of products in reasonable reaction times. In contrast, in the absence of any catalyst in water, this reaction afforded compound 4a after 60 min in very low yield (< 30%).
To evaluate the scope and generality of this protocol, different aromatic aldehydes and barbituric acids were used in the presence of SBA-Pr-SO3H as the catalyst. The results are summarized in Table 2. Corresponding pyrazolopyranopyrimidines were successfully prepared in high to excellent yields. As shown in Table 2, both electron-rich and electron-deficient aldehydes gave pyrazolopyranopyrimidines in excellent yields and very short reaction times. Melting points were compared with reported literature values (Table 2). The generated solid products were dissolved in hot ethanol and acetone (1:1), and separation of the heterogeneous solid catalyst from the reaction medium was easily carried out by simple filtration.
The reactions were monitored by TLC. The generated solid product was dissolved in hot ethanol and acetone (1:1), filtered for removing the catalyst and then the filtrate was cooled to afford the pure crystals of pyrazolopyranopyrimidines.
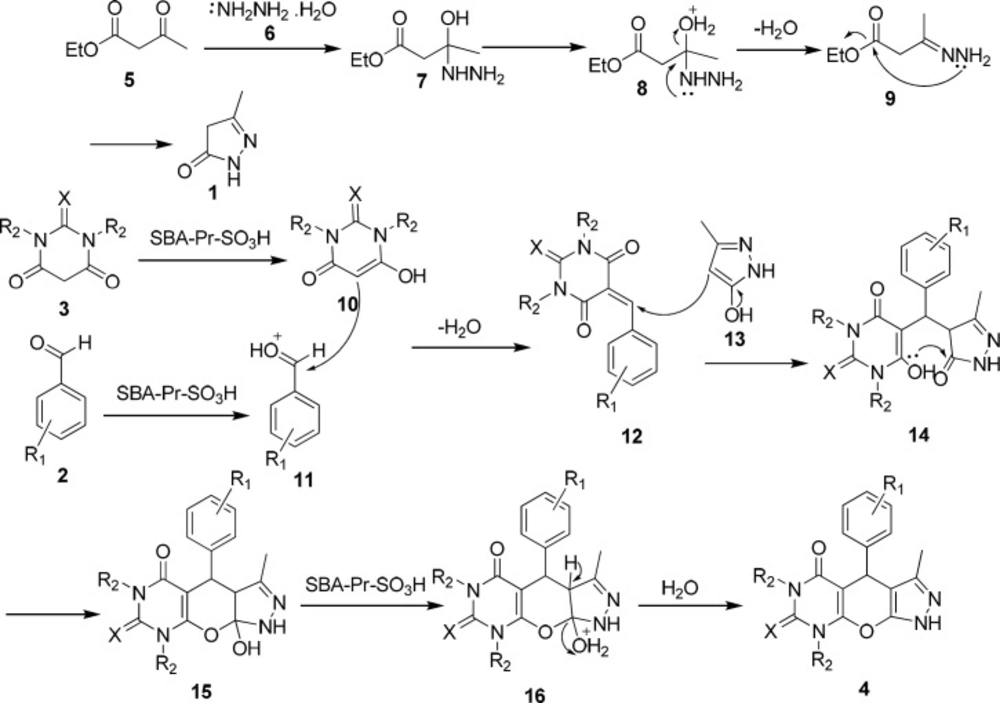
A possible mechanism for the formation of products 4a-i in the presence of SBA-Pr-SO3H is shown in Scheme 2. At first, 3-methyl-5-pyrazolone 1 was obtained through the nucleophilic attack of hydrazine hydrate 6 to ethyl acetoacetate 5 to form an imine intermediate 9 which further undergoes intermolecular condensation to form 3-methyl-5-pyrazolone 1. In the main reaction, protonation of the carbonyl group of benzaldehyde 2 by the solid acid catalyst activates it toward nucleophilic attack of barbituric acid enole form 10 to yield intermediate 12. Subsequently, the Michael addition of 3-methyl-5-pyrazolone enolic form 13 to compound 12 results in the formation of intermediate 14 which by the cycloaddition of hydroxyl group to the carbonyl group produces compound 15. Finally, elimination of water affords the corresponding pyrazolopyranopyrimidine 4.
Table 3 compares the effectiveness of various catalysts used in the synthesis of pyrazolopyranopyrimines. The results demonstrate that the present methodology is more efficient and less time-consuming when compared with other methods.
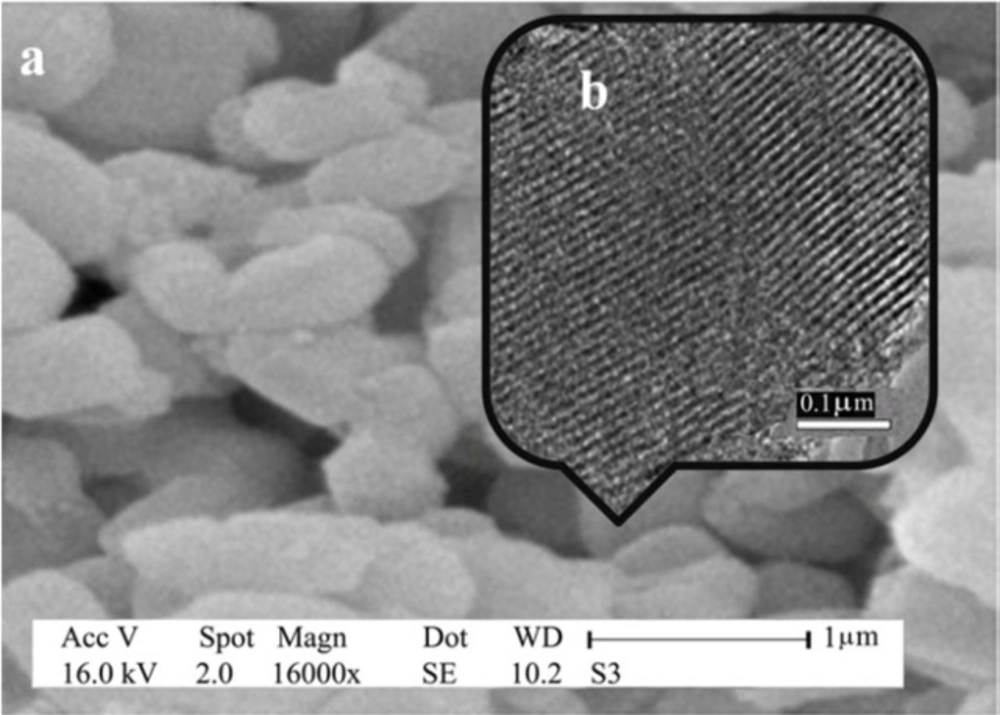
The morphology of SBA-Pr-SO3H was verified by SEM and TEM images (Figure 2). The results confirmed that the hexagonally ordered mesoporous structure of SBA-15 silica was well retained after the chemical grafting reaction. The TEM image (Figure 2b) showed the uniform and parallel channels, which were open along the particles.
The inhibition zones of compounds around the discs are shown in Table 4. As can be seen, all compounds exhibited significant antibacterial activities against S. aureus and C. albicans when compared with the reference drugs. All compounds were able to inhibit the growth of B. subtilis and E. coli. Figure. 3 illustrates the inhibition zones of compounds around the disks with B. subtilis. No compound showed antibiotic activity against P. aeruginosa. Table 5 illustrates the minimum inhibitory concentration (MIC) of the synthesized compounds. The screening results indicated that among the synthesized products, compounds 4a (R1 = 4-Cl, R2 = H), 4f (R1 = 4-Cl, R2 = Me), and 4h (R1 = H, R2 = Me) exhibited significant antibacterial activities at low concentrations, whereas the other compounds generally showed lower activities. The best result was observed for C. albicans which was sensitive to compounds 4a, 4f and 4h with MIC being between 2 and 8 µg/mL.
Conclusion
In conclusion, we have developed an efficient, clean and simple procedure for the synthesis of pyrazolopyranopyrimidine derivatives in excellent yields in the presence of catalytic amount of SBA-Pr-SO3H as an environmentally benign solid acid catalyst. High yields, short reaction times, convenient work-up procedure, and the use of water as a solvent are the advantages of this method. Compounds synthesized by this method were screened for their antimicrobial activities and products 4a, 4f and 4h showed significant antimicrobial activities against some fungi and gram positive and gram negative bacteria.