Introduction
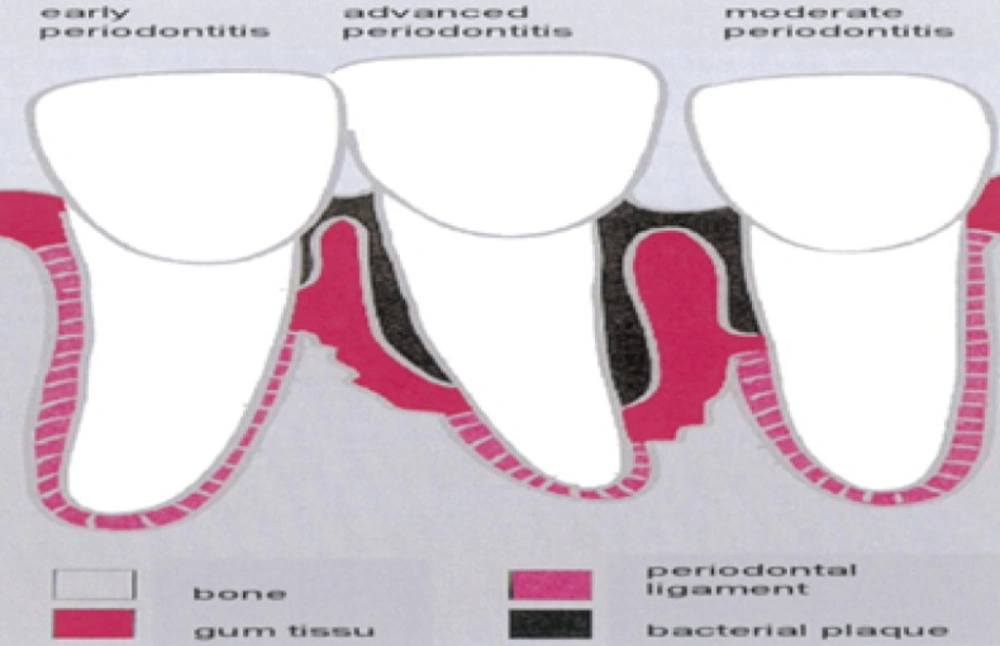
Dental diseases are among the most widespread chronic disorders affecting mankind. The high incidence of carries and periodontal diseases is borne out by epidemiological studies carried out in many parts of the world. Compared to the widespread use of drugs for treating many medical conditions, relatively little use has been made of such forms of therapies in dental settings. Although employed to allay pain and anxiety and with the exceptions of fluoride, drugs have not been extensively used for treating dental diseases. Periodontal diseases are groups of infections and inflammatory conditions, including gingivitis and periodontitis that affect teeth supporting structures. These diseases occur when bacteria from dental plaque invade surrounding tissues and from the accumulation of plaque at the gingival margin, which, in turn, induces an inflammatory response (1).
The most common form of periodontal disease is referred to as chronic periodontal disease describinga group of related inflammatory reactions resulting in destruction of the tissues that support tooth in its pocket. There are several types of periodontal diseases (periodontitis) which include chronic periodontitis, aggressive periodontitis, systemic disease associated periodontitis, and necrotizing periodontitis. The clinical signs of periodontitis are changedin the morphology of gingival tissue, bleeding upon probing as well as periodontal pocket formation. This pocket provides an ideal environment for the growth and proliferation of anaerobic pathogenic bacteria. Periodontal diseases are group of conditions, including gingivitis and periodontitis, which affect the supporting structures of the teeth such as gums, periodontal ligaments, alveolar bone, and dental cementum. It is a localised inflammatory response caused by bacterial infection of a periodontal pocket associated with subgingival plaque (2). Although the bacteria are the primary cause, the expression of microbial pathogenic factors alone may not be sufficient to cause periodontitis but they initiate damage directly or indirectly by triggering host-mediated responses that lead to self-injury. This disease caused by bacterial infection of a periodontal pocket arising from the accumulation of subgingival plaque and inflammatory response, which can result in tooth loss (3). Various intra-pocket drug delivery systems have been proposed, including fibers, strips, films, injectable gels, and micro particles (4). Periodontitis cannot be cured, but it can be arrested. It can be localized or generalized and depending on the amount of clinical attachment loss, the severity of the disease process is labelled as mild (< 3mm) moderate (3-4mm) or severe (5mm). The local drug delivery to be effective, it must meet three requirements: It must: (a) reach the sites of disease activity namely the base of the pocket (b) be delivered at a bacteriostatic bacteriocidal concentration and (c) remain in place long enough to be effective.
Many polymer-based intra- pocket devices containing drugs for this treatment had been studies as tetracycline loaded into poloxamer and monoglycerides (6). Tetracycline-loaded bioadhesive semisolid, polymeric system based upon hydroxylethylcellulose and polyvinyl pyrrolidone (8) and Metronidazole-loaded systems based upon Carbopol 974P, hydroxyl ethyl cellulose and polycarbophil (7) revealed significant results. Tetracycline base loaded into the micro tubular excipient halloysite and coated with chitosan to further retard the drug release (9). Intra-pocket delivery devices in the periodontal pocket are of following types such as fibres (10) strips (11) films (12) and injectable gels (13). The various drugs were utilised for periodontal therapy such as chlorhexidine, tetracycline, minocycline and metronidazole. The reason behind this apparent omission is of interest in the context of local delivery approach. The effectiveness of dental drugs has been generally evaluated only for conventional modes. To improve this method of drug administration the aims and optimal duration of treatment need to be further clarified so that development of delivery system can occur through modification of the device›s release and degradation characteristics.
Experimental
Material
Doxycycline hyclate was obtained as a gift samples from Zydus cadila healthcare Pvt. Ltd Ahmadabad, India.Pluronic F127 and Zinc oxide was purchased from Rankem chemicals Mumbai India, N-methyl-2-spyrolidione the other materials such as sodium hydroxide, Potassium dihydrogen phosphate, sodium chloride, calcium chloride, hydrochloric acid, methanol was purchased from Loba chemicals, Pvt. Ltd Mumbai India. Double distilled water was used throughout the study.
Methods
Physiochemical Compatibility Studies of Drug and Polymer
Differential Scanning Calorimetry (DSC)
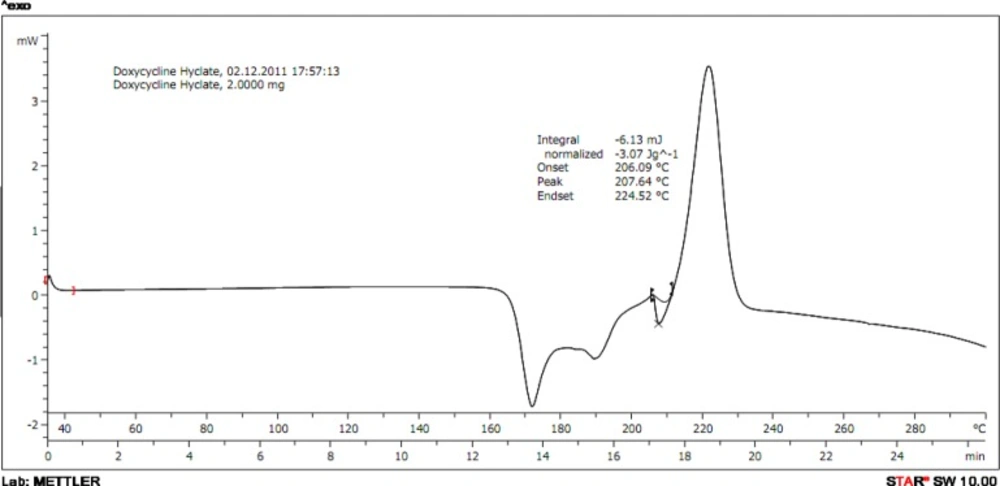
The melting temperature of drug was determined using capillary method and confirmed by differential scanning calorimetry (DSC). Thermogram of DH was obtained using DSC (DSC 822c, Mettler Toledo). To checkDrug-Polymer interaction study by DSC the drug and polymer taken then hermetically sealed in perforated aluminium pans and heated at constant rate of 10 °C/min over the temperature ranges of 35-300 °C.
Calibration Curve in pH 7.4 Phosphate Buffer
10 mg of DH was dissolved in 100 mL of pH 7.4 buffers to obtained working standard of 100 μg/mL Aliquots of 0.4 mL to 2.8 mL from the stock solution representing 04 to 28 μg/mL of drug transferred to 10 mL volumetric flask and also the volume was adjusted to mark. Absorbance of the above solution was taken at 273 nm by UV spectrophotometer (1700, Shimadzu, Japan) against the blank solution prepared in the same manner without adding the drug. A graph of absorbance Vs concentration was plotted from above obtained readings.
Formulation and development
A) Preparation of in situ-gel
Formulations were prepared on a weight basis using a cold process. Carefully a weighed amount of pluronic F127 sufficient to yield was slowly added to cold water (5°C). DH was added with a constant stirring, the dispersion was then refrigerated until a clear solution was formed (i e upto 5 h). Active substances like zinc oxide which is insoluble in water was dissolved prior to addition in N-methyl 2-pyrolidone (5°C) to form a homogeneous mass. Then prepared formulations were stored in well closed amber coloured container until it is used. Following batches were developed which contain the appropriate concentration of drug and various concentration of excipients.
Physical properties
The appearances of formulations were inspected by visual observation. The observed clarity and colour of formulated gel was determined by visual inspection under black & white background.
Drug content
Formulations containing 1 mg drug was taken in 10 mL volumetric flask dissolved in methanol then made up the volume to 10 mL then filtered. Absorbance values were measured by UV-spectrophotometer (1700, Shimadzu, Japan) at λmax 273 nm. Concentrations of drug were calculated from the standard calibration curve prepared in methanol to get the drug content (14).
Where, As: absorbance of sample solution,
GL: conc. of drug in standard solution,
Gr: absorbance of standard drug solution.
Surface pH
An acidic or alkaline formulation was bound to cause irritation on mucosal membrane and hence this parameter assumes significance while developing a mucoadhesive formulation. A digital glass electrode pH meter was used for this purpose. pH was noted by dipping the electrode in to the formulations and allow to equilibrating for 1 min (15).
Gelation & Gel melting temperature
Typically, the determination of the boundary between the sol and gel phases depends on the selected experimental method. In this study a simple test-tube inverting method was employed to roughly determine the phase boundary. Gelation and gel melting temperatures were assessed using a modification of the Miller and Donavan technique. A 5 mL aliquot of gel was transferred to test tubes and immersed in a thermostat water bath at 4 °C, and sealed with aluminium foil. The temperature was increased with the increments of 1 °C and left to equilibrate for 1 min at each new setting. The samples were then examined for gelation, which was said to have occurred when the meniscus would no longer move upon tilting through 90 °C. The gel melting temperature, the temperature at which a gel started flowing upon tilting through 90 °C, was recorded (16).
Rheological study
Viscosities of formulations before and after gelation were measured by using Brook field DV-E viscometer using Spindle number-3 at 100 rpm shear rate. In case of Pluronic F127 based systems the viscosity was recorded at increasing temperature in range of 20 °C to 30 °C and graphs were plotted. From which an approximate gelation temperature was determined as indicated by sudden rise of viscosity (17).
Syringebility study
All the developed formulations were tested by using 21-gauge needle for the intention whether the formulations passes through it (8).
Mucoadhesive Strength
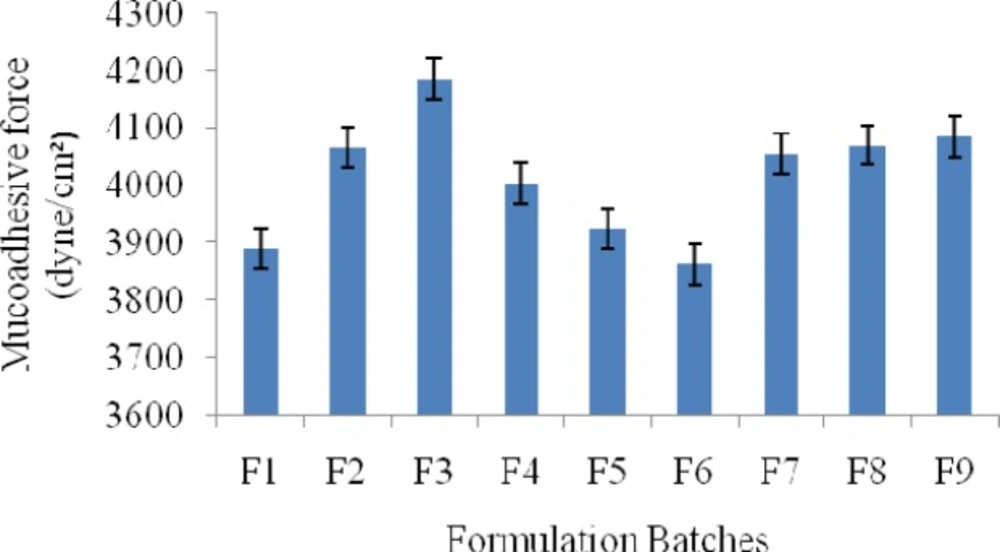
The mucoadhesive potential of each formulation was determined by measuring the force required to detach the formulation from sheep intestinal membrane using a modified physical balance method; results were shown in Figure 4. In brief, intestinal membrane was carefully removed from the instestinal cavity of sheep obtained from the local slaughterhouse. Tissues were immediately used after separation. At the time of testing, a section of intestinal membrane tissue was secured (keeping the mucosal side out) to the upper probe using a cyanoacrylate adhesive. The surface area of each exposed mucosal membrane was 4.2 cm2. At room temperature, fixed amount of samples of each formulation were placed on the lower probe. The probes were equilibrated. Probe with intestinal tissue was lowered until the tissue contacted the surface of the sample. Immediately, a slight force was applied for 2 min. to ensure intimate contact between the tissue and the sample. The mucoadhesive force, expressed as the detachment stress in dyne/cm2, was determined from the minimal weights that detached the tissues from the surface of each formulation using the following equation (13).
Where, m - The weight added to the balance in grams,
g - The acceleration due to gravity taken as 980 cm/s2
A - Surface area of sheep intestinal mucosa
In-vitro drug release study
The release studies were performed using the dialysis method. Typically 1g of pluronic F127 gel was placed in a dialysis tube (having MW 12000 Daltons). The dialysis tube (having 2.3cm in diameter) was then placed in a vessel containing 100 mL of buffer solution pH 7.4, (simulated pH of GCF) stirred at 100 rpm. Samples were collected periodically and replaced with fresh medium of phosphate bufferpH 7.4. After filtration through the Whatman filter paper no. 41, the concentration of DH was determined by UV- spectrophotometry method 273nm.
Anti-microbial activity
The antimicrobial efficiency and prolonged effect of selected controlled release Doxycycline hyclate in-situ gel were determined on S. aureus, E. coli, and C. albicans strains as a function of time. The inhibitory effect of formulation on the studied microorganisms was evaluated using agar well diffusion test. Wells were punched into the nutrient agar previously seeded with test organisms and they were filled with 100 μL of the samples. After allowing diffusion of solution for 2 h the plates were incubated for 24 h at 37 °C and the diameters of inhibition zones were measured. The inhibitory effect of optimized gel formulation was compared with marketed Doxycycline hyclate dental gel (20).
Results and Discussions
Results
Melting point of DH was measured; and found to be in the range of 205-208 °C. It was confirmed with the reported melting point of DH. It was also confirmed by differential scanning calorimetry at scanning rate of 10 °C/min and it exhibits a sharp melting endothermic peak at temperature of 207.64 °C.38
Drug polymer compatibility study of pure drug, drug-polymer physical mixtures were analyzed by IR spectroscopy and Differential Scanning Calorimetry indicates that there were no interation between the excipients added and drug hence suitable for formulations. The thermogram of physical mixture of DH and Pluronic F-127; ratio of 1:1 were taken which showed no shifting in the thermograms proving that there were no drug-polymer interaction.
Bacteria in subgingival plaque have caused a periodontal pocket to develop, inflaming surrounding tissue and causing loss of alveolar bone (5).
| Sr No. | Formulation | Doxycycline hyclate(%w/w) | Pluronic F127(%w/w) | NMP(%w/w) | ZnO(%w/w) | Purified Water |
|---|---|---|---|---|---|---|
| 1 | F1 | 5 | 18 | - | - | q.s |
| 2 | F2 | 5 | 19 | - | - | q.s |
| 3 | F3 | 5 | 20 | - | - | q.s |
| 4 | F4 | 5 | 20 | 10 | - | q.s |
| 5 | F5 | 5 | 20 | 20 | - | q.s |
| 6 | F6 | 5 | 20 | 30 | - | q.s |
| 7 | F7 | 5 | 20 | 20 | 0.1 | q.s |
| 8 | F8 | 5 | 20 | 20 | 0.2 | q.s |
| 9 | F9 | 5 | 20 | 20 | 0.3 | q.s |
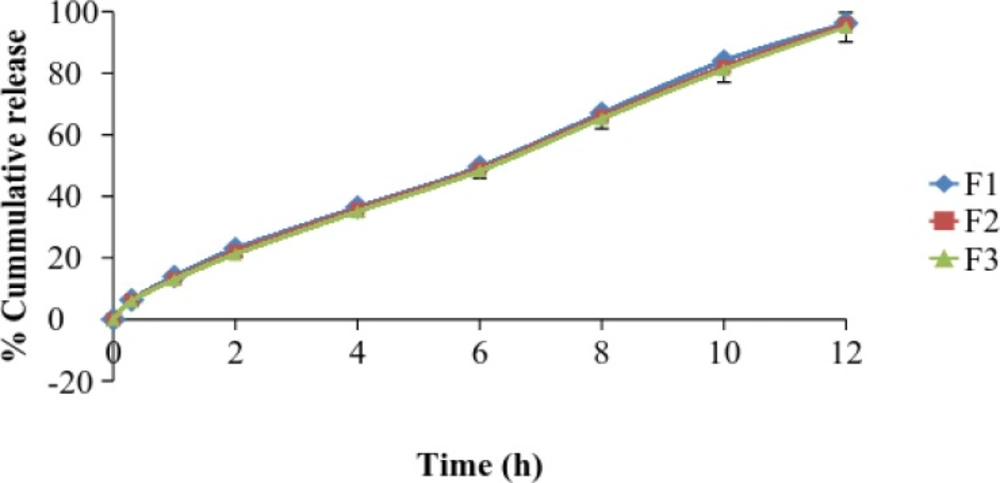
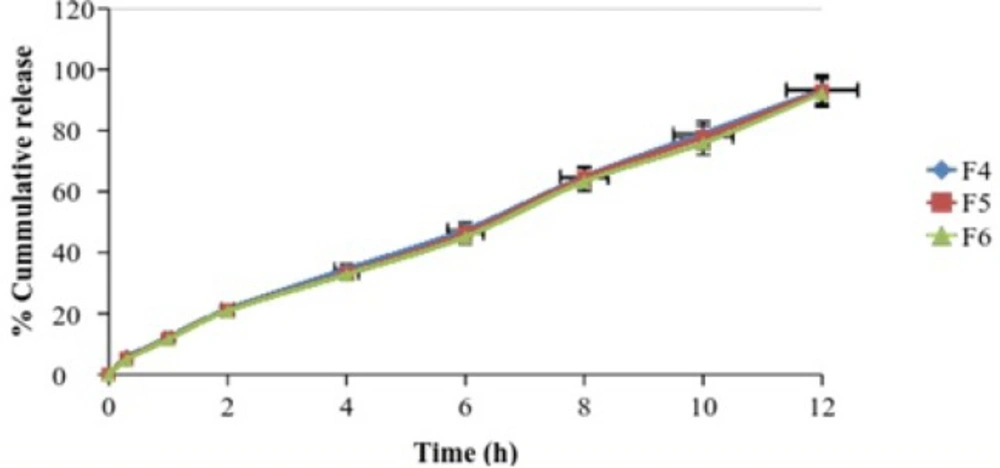
| Time (h) | % Cumulative drug release (Mean ± S.D.) | ||
|---|---|---|---|
| F4 | F5 | F6 | |
| 0 | 0 | 0 | 0 |
| 0.3 | 5.51 ± 1.4 | 5.12 ± 1.0 | 4.9 ± 1.4 |
| 1 | 12.16 ± 1.2 | 11.8 ± 1.8 | 11.54 ± 2.1 |
| 2 | 21.37 ± 2.1 | 21 ± 2.2 | 20.87 ± 2.4 |
| 4 | 34.56 ± 1.8 | 33.26 ± 1.4 | 32.79 ± 3.2 |
| 6 | 47.47 ± 2.4 | 46.34 ± 3.0 | 45.15 ± 1.9 |
| 8 | 64.9 ± 3.1 | 64.43 ± 2.3 | 63.27 ± 1.7 |
| 10 | 79.04 ± 2.6 | 77.82 ± 2.7 | 75.88 ± 2.1 |
| 12 | 93.51 ± 1.8 | 92.97 ± 2.4 | 92.21 ± 2.3 |
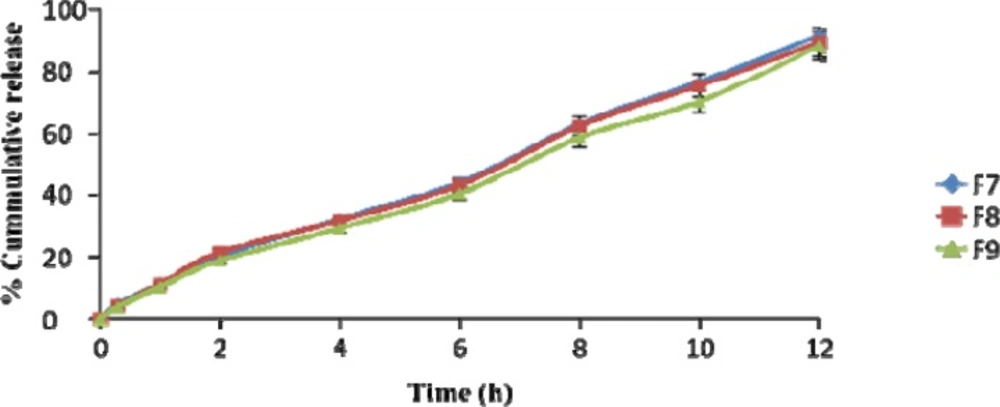
| Time (h) | % Cumulative drug release (Mean ± S.D.) | ||
|---|---|---|---|
| F7 | F8 | F9 | |
| 0 | 0 | 0 | 0 |
| 0.3 | 4.67 ± 1.4 | 4.11 ± 1.7 | 3.83 ± 2.1 |
| 1 | 11.07 ± 1.8 | 10.87 ± 2.3 | 10.04 ± 1.9 |
| 2 | 20.31 ± 1.6 | 21.43 ± 2.6 | 18.67 ± 1.5 |
| 4 | 32.06 ± 2.1 | 31.6 ± 1.4 | 29.23 ± 1.1 |
| 6 | 43.74 ± 3.1 | 42.95 ± 1.7 | 40.21 ± 2.7 |
| 8 | 62.63 ± 2.7 | 62.13 ± 2.9 | 58.43 ± 3.1 |
| 10 | 76.33 ± 2.4 | 75.13 ± 2.1 | 69.94 ± 1.4 |
| 12 | 91.38 ± 1.5 | 89.24 ± 1.8 | 88.16 ± 2.2 |
| Sr. no | Batches | Inhibition zone (mm) (Mean ± S.D.) | ||
|---|---|---|---|---|
| S. Aureus | E. Coli | C. Albicans | ||
| 1 | F1 | 11.5±0.2 | 10.3±0.3 | 9.3±0.2 |
| 2 | F2 | 10.7±0.5 | 9.4±0.6 | 8.9±0.6 |
| 3 | F3 | 10.3±0.7 | 9.5±0.4 | 8.4±0.3 |
| 4 | F4 | 11.2±0.8 | 9.2±0.7 | 8.3±0.6 |
| 5 | F5 | 11.4±0.5 | 9.6±0.2 | 7.5±0.7 |
| 6 | F6 | 10.7±0.4 | 9.7±0.5 | 7.1±0.5 |
| 7 | F7 | 21.9±0.6 | 18.8±0.7 | 16.3±0.6 |
| 8 | F8 | 24.4±0.1 | 21.9±0.9 | 19.7±0.4 |
| 9 | F9 | 27.7±0.7 | 24.5±0.7 | 20.2±0.7 |
| Formulation code | R2 | Best fit model | ||
|---|---|---|---|---|
| Zero order | First order | Higuchi model | ||
| F1 | 0.995 | 0.862 | 0.953 | Zero order |
| F2 | 0.996 | 0.860 | 0.951 | Zero order |
| F3 | 0.997 | 0.872 | 0.949 | Higuchi model |
| F4 | 0.997 | 0.890 | 0.950 | Higuchi model |
| F5 | 0.997 | 0.890 | 0.947 | Zero order |
| F6 | 0.996 | 0.889 | 0.945 | Zero order or Higuchi model |
| F7 | 0.996 | 0.899 | 0.941 | Zero order or Higuchi model |
| F8 | 0.995 | 0.919 | 0.944 | Zero order or Higuchi model |
| F9 | 0.995 | 0.898 | 0.931 | Zero order or Higuchi model |
| Formulation code | R2 | Mechanism | ||
|---|---|---|---|---|
| ‘k’ value | ‘n’ value | R2 | ||
| F1 | 1.154 | 0.738 | 0.995 | Anomalous (Non-Fickain) transport |
| F2 | 1.137 | 0.749 | 0.994 | Anomalous (Non-Fickain) transport |
| F3 | 1.126 | 0.754 | 0.994 | Anomalous (Non-Fickain) transport |
| F4 | 1.108 | 0.768 | 0.995 | Anomalous (Non-Fickain) transport |
| F5 | 1.189 | 0.783 | 0.995 | Anomaloas (Non-Fickain) transport |
| F6 | 1.077 | 0.788 | 0.996 | Anomaloas (Non-Fickain) transport |
| F7 | 1.061 | 0.800 | 0.995 | Anomaloas (Non-Fickain) transport |
| F8 | 1.041 | 0.820 | 0.995 | Anomaloas (Non-Fickain) transport |
| F9 | 1.004 | 0.832 | 0.995 | Anomaloas (Non-Fickain) transport |
Formulations were prepared using cold method. Antibacterial drug such as DH was used in the formulations. Total nine batches were prepared in Batch B1, B2 onlythe pluronic was used in the various concentrations, but according to their physical appearance, gelation and sol to gel formation phenomenon only 20% w/w was considered as an optimum concentration. Formulations B4, B5 and B6 were prepared by utilizing NMP at a range of 10 & 30% concentration, but 20% w/w was found to be best concentration and therefore was selected for further studies. NMP was added because it forms los viscous solution in presence of polymer.
Formulations containing xanthan gum viz., B3, B6, and B9 were prepared in concentration 0.1 to 0.3% w/v. Optimum concentration 0.3% w/v was found to be best. pH of formulations was adjusted between 6.2 to 6.8 pH; by using phospate buffer. The formulations were filled in 10 mL amber colored glass vials, capped with rubber bungs and sealed with aluminium caps. Formulations were stored in a refrigerator (4–8 °C) until use. The prepared in situ gels containing Doxycycline hyclate was found to be transparent, clear and the colour of formulation was found to be transparent. The drug content of the formulation was found to be in the range of 87.15% and 94.21%. So, on that basis we can say that the drug was uniformly distributed throughout the formulations.
The speed and extent of gelation for all formulations were determined. The temperature taken to form the gel was in the range of 30.6 to 37.4 °C. Physically, gel formation is related to micellar packing and volume fraction. Researchers have attributed gelation to the dehydration of PPO groups in the micelle core, a change in the micellar volume or a decrease in the critical micelle concentration and an increase in the aggregation number (18). The finding that tetracycline lowered the gelation temperature was similar to the result reported by Esposito et al., 1996 (6). This feature was tentatively explained by a facilitation of the interaction between the hydrophobic portion of the polymer molecules, which could disrupt the micellar structure and increase the entanglement of micelles. Pisal et al., 2004 have also reported a significant decrease in gelation temperature by vitamin B12 at all concentrations of pluronic F 127, attributed to the higher water solubility of vitamin B12. The presence of vitamin B12 predominantly decreased the critical micellar concentration and increased the micellar aggregation (19). While the temperature required for melting the gel containing NMP was found to be in the range of 82.8 to 79.4 for 10 to 30% NMP. While the ZnO concentration increases 0.1 to 0.3%, it was found that both the gelation temperature and gel melting temperature decreased from 26.3 to 22.5 and 78.5 to 75.8.
The apparent viscosity values were measured for liquid formulations and gel using Brookfield viscometer DV-E with spindle no. 3 at 100 rpm. The marked increase in viscosity of the in situ gel formulations was increased from 6421.0 to 6978.9 cps as the Pluronic F 127 concentration was increased from 10 to 30%. It was also seen that the viscosity of the gel formulation was decreased from 6379.3 to 6032.7 cps as the concentration of NMP increased from 10 to 30%. While addition of ZnO, it was directly proportional to the viscosity of gel formulations i.e., increase in the concentration of ZnO gradually increases the viscosity of gel formulations i e from 6165.0 to 6187.1 cps; was found to be in proportionate with the increasing polymer concentration.
All the prepared formulations were found to be syringeable through 21-gauge needle. Results revealed that all the formulations (F1-F9) were found to be syringeable at cold temperature.
Mucoadhesive Strength (MS) of various formulations was carried out; mainly to select the polymer proportion and total polymer concentration for the prepared gels. The results of mucoadhesive strength measurement by modified physical balance method are shown in Figure. 8. It was found that, as the total concentration of polymers (Pluronic F-127) increased, the mucoadhesive strength (MS) was found to increase. The mucoadhesive material must come in close contact with the tissue for the mucoadhesion. Pluronic F127, due to their chemical nature, is high-molecular-weight polymer that readily swells in water; the swelling exposes a large adhesive surface for maximum contact with themucosa (the glycoprotein predominant in the mucous layer) and, thus, provides excellent mucoadhesion. The stronger the bioadhesive force more is the residence time. But if the mucoadhesion is too strong the gel can damage the mucosal membrane. In this study, it was found that as the concentration of NMP increased from 10 to 30% the MS of the prepared gel decreased profoundly mainly because of the solvent nature of NMP which decreases both the viscosity and the MS. The effect of ZnO over the MS was not much significant which can be shown in Figure.
In-vitro drug release profile provides insight into the efficiency of the drug delivery system proposed for the controlled release of drug.
The drug release profile was tested in phosphate buffer solution pH 7.4 to imitate the environment of periodontitis. Systems containing 5 %w/w DH & 20%w/w Pluronic F127 showed a release of drug 45% in the period of 5 h. It has been suggested that Pluronic F127 might retard the release of drug since the structure of the gel functioned as barrier to the release of drug.
The influence of NMP on release of drug from the formulation containing 20% w/w Pluronic F127 and DH was also investigated. The added 20%w/w NMP into the gel systems enhanced the release of DH. This result signified that NMP might be decrease the gel strength of the formulation.
DH released from formulation shows a biphasic characteristic, with a relatively fast release at the initial hours and then release of drug was almost completed. The amount of drug released from the same batch was found to be 90% at the end of 12 h.
Drug release kinetics
Model fitting to drug release profile (18)
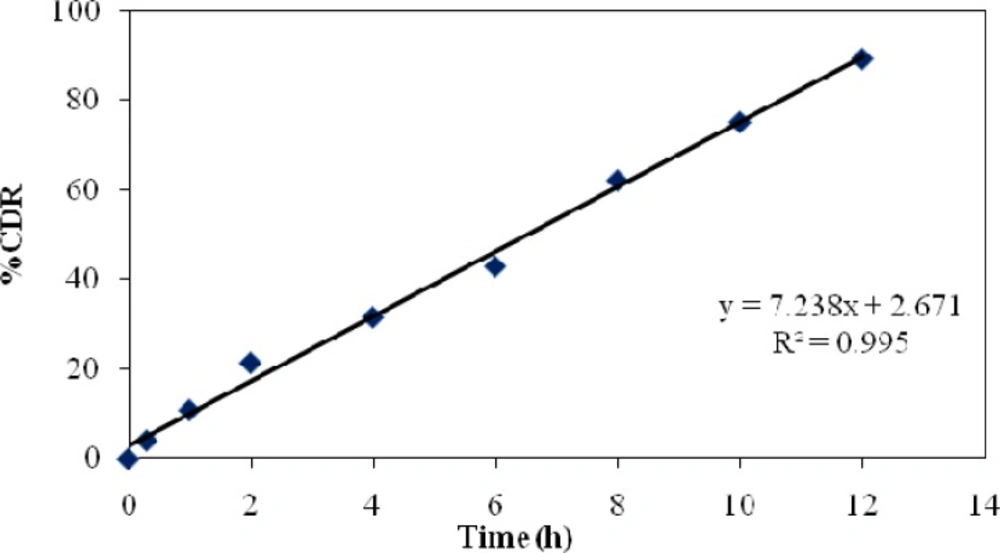
The release constant was calculated from the slope of the appropriate plots, and the regression coefficient (R2) was determined. It was found that the in-vitro drug release of formulation was best explained by zero order, as the plots showed the highest linearity.
Mechanism of drug release
The corresponding plot of (log cumulative percent drug release Vs log time) of the Korsmeyer-Peppa’s equation indicated a good linearity of regression.
Discussion
To improve this method of drug administration the aims and optimal duration of treatment needs to be further clarified so that development of delivery system can occur through modification of the device›s release and degradation characteristics. In Situ gels so prepared and characterized for its physical properties, drug content, pH, gelation properties, gelation strength, rheology, syringebility, mucoadhesion, anti- microbial activity, in-vitro drug release study, and their kinetic rate profile. Physical appearance of formulations was found to be clear and transparent.
The drug content of formulation was found to be satisfactory& in the ranges of 87.15 to 94.21%. The pH of formulations was found to be in the ranges of 4.58 to 5.38. Gelation and Gel melting temperature of formulations was found to be in the ranges of 22.5 to 37.4 °C and 75.8 to 86.7
°C, respectively. Viscosity of formulations was found to be in the ranges of 32991 to 37294 cps. All the prepared formulations were found to be syringeable through 21-gauge needle. Mucoadhesive strength of formulations was found to be in the ranges of 3889 to 4186 dyne/cm2. Results of antimicrobial activity was found to be against S. Aureus in the ranges of 10.3 to 27.7 mm, E.Coli in the ranges of 9.2 to 24.5 mm and C. Albicans 7.1 to 20.2 mm respectively. In-vitro drug release profile was found to be in the ranges of 88.16 to 96.29% at the end of 12 h. The model fitting to drug release profile was zero order for F1, F2 and F5 batch, Higuchi model for F3-F4 batch and zero order or higuchi model for F6-F9 batch.
The mechanism of drug release was anomalous (non-fickain) transport for all the prepared batches. From the aforesaid studies, formulation F8 was optimized and further subjected for texture analysis for studying the hardness, compressibility, adhesiveness, cohesiveness, and DSC. Formulated in situ gels F6 showed the effective pH, gelation, viscosity, gel strength, and drug release properties along with good mucoadhesive strength. The drug release mechanism from the gel matrices was found to be anomalous and following the Zero order and also gives the satisfactory result of Texture analysis and DSC. The results of present study clearly indicated promising potential of in situ gel containing Doxycycline Hyclate for delivering drug to infected periodontal pockets and could be viewed as alternative to conventional dosage form.
In conclusion, the formulated in situ gel as a drug delivery system promising the approach which are utilised for improving therapeutic efficacy of Doxycycline hyclate in the treatment of periodontitis. The use of Pluronic F127 as well as NMP showed better drug release profile at the end of 12 h, ZnO showing a better antibacterial activity. Once administered, the solvent diffuses out leading to the formation of depot for controlled delivery of active ingredient. The concentration of polymer, type of solvent, and additives used affect release rate of drug from depot formed in situ. Inclusion of ZnO also improve therapeutic efficacy of formulation in the management of periodontitis. Hence, in future such type of drug delivery system may utilise for the periodontal treatment.