Introduction
Rituximab, a chimeric mouse/human monoclonal antibody (mAb) (2, 3, 4) coupled with beta emitting radionuclides, enhances therapeutic effectiveness of anti-CD20 (5, 6, 7). Such radioimmunoconjugates bind to CD20 antigen, a transmembrane phosphorylated protein, located on pre-B and matured B-lymphocytes and more than 90% of B-cell Non-Hodgkin’s Lymphomas (NHL) (8, 9, 10).
Pre-clinical and clinical applications of 131I- (11), 99mTc- (12), 90Y- (13, 14, 15), or 177Lu-labeled (16) Rituximab have been already reported. Many reports described the radiolabelling of anti-CD20 with 177Lu and 90Y after conjugation with DOTA or DTPA as a bifuncional chelating agents because they form very stable complexes (17, 18). However, chemical modification of antibodies may alter their chemical or biological properties. Based on the recent experiments on the kit formulation of DOTA-Rituximab conjugate (1), our present study is focused on comparative biodistribution of radioimmunoconjugates prepared after conjugation of Rituximab with DOTA-(SCN) or DOTA (NHS) chelator. The biodistribution is an essential preclinical procedure to assess the localization of radioligand. It allows determining the off-target accumulation and clearance rate (19). To achieve this, organs and tissues are excised from animals (usually mice and rats) at indicated time points and analyzed. This is laborious and involves the use of many animals. In order to preliminarily and non-invasively assess the 90Y-DOTA-Rituximab biodistribution in-vivo, we used optical imaging. Numerous studies have demonstrated that for a variety of β+, β- radionuclides bound up with peptides, antibodies and nanoparticles enable optical imaging due to Cerenkov light emission (20, 21).
Herein we report the influence of two DOTA precursors, p-SCN-Bn-DOTA and DOTA-NHS-ester conjugated to Rituximab and radiolabelled with 177Lu and 90Y on radiochemistry and biodistribution in tumour-bearing mice.
Experimental
Antibody and reagents
Rituximab (MabThera®) was purchased from Roche Pharma AG, Germany. The bifunctional chelators: p-SCN-Bn-DOTA(2-(4-isothiocyanatobenzyl)-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) and DOTA-NHS-ester (1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid mono (N-hydroxysuccinimide ester) were purchased from Macrocyclics. Both radionuclides in the chemical form of chloride in 0.04 M HCl, i.e.: Lutetium-177 (LutaPol) of SA higher than 555 MBq/mg Lu, and non-carrier added Yttrium-90 (ItraPol) were produced at Radioisotope Centre POLATOM, Poland. All other chemicals and materials were used as supplied and were of the highest purity available. For all procedures HPLC grade water was used to avoid metal contamination.
Synthesis of the DOTA-Rituximab conjugates
Two derivatives of DOTA bifunctional chelating agent, i.e. DOTA-SCN and DOTA-NHS, were conjugated to anti-CD20 antibody (Rituximab) and finally the conjugates were formulated in the form of a freeze-dried kit as previously described (1). Briefly, the concentrated Rituximab (20 mg in 2 mL) was incubated with a 10-fold molar excess of DOTA-SCN chelator at 37 °C for 1.5 h in the carbonate buffer with gentle mixing. An excess of the unreacted chelator was removed by ultrafiltration (Amicon Ultra; MWCO 30,000; Millipore). For preparation of Rituximab conjugate with DOTA-NHS-ester the protocol developed within the IAEA project with slight modifications was used (22). The solution of anti-CD20 (Rituximab; 10 mg/mL) was initially purified by ultrafiltration using an Amicon centrifuge filter Ultra-2mL (30 min, 5000 rpm). The concentrated mAb was incubated with 40mM of DTPA at 4 °C for 30 min and then loaded on the Sephadex G-25 column (PD-10) and eluted with a 0.05 M phosphate buffer of pH 7.0. The 0.5 mL fractions with the highest concentration of mAb (quantified calorimetrically with the Bradford method) were collected and incubated with 100-fold molar excess of DOTA-NHS-ester at 4 °C with gentle stirring for 24 h. The reaction mixtures were transferred on an Amicon centrifuge filter to exchange the buffer to 0.5 M ammonium acetate of pH 5.5 and to remove the excess of unbound DOTA-chelator. The concentration of the antibody in the final immunoconjugate solution was measured by the Bradford method. The average number of DOTA molecules coupled per mAb molecule for both chelators were determined by radiolabelling assay using 64Cu (23, 24).
Radiolabelling
The immunoconjugates were radiolabeled with 177Lu and 90Y as follows: the freeze-dried kit containing 2.0 mg of DOTA-anti-CD20 was dissolved in 0.5 mL of water and mixed with 100-900 MBq of [177Lu]LuCl3 or 90YCl3. The solutions were incubated at 37 °C for 1h. The quality control of the resulting radioimmunoconjugates was performed by ITLC-SG with methanol/0.4 M ammonium acetate (1:1, v/v) as a mobile phase and by size exclusion high performance chromatography (SE-HPLC) using BioSep-SEC-S3000 PEEK column (300x7.5 mm; Phenomenex) eluted with a 0.1 M phosphate buffer of pH 5.8 at a flow rate of 1 mL/min. The radiochemical purity (RCP) and stability of 177Lu-DOTA-Rituximab and 90Y-DOTA-Rituximab were evaluated in the presence of an excess of competitor such as 10mM DTPA. Due to a high RCP of the 90Y- and 177Lu- DOTA-Rituximab obtained, no additional purification step was necessary.
Stability of 90Y-DOTA-Rituximab and 177Lu-DOTA-Rituximab in human serum and in 0.9 % NaCl was determined after 1, 24, 48, and 72 h of incubation using SE-HPLC and ITLC-SG radioanalytical techniques. To investigate stability in human serum, 200 µL of 177Lu or 90Y-immunoconjugates were incubated in 0.5 mL of fresh human serum up to 72 h at 37 °C.
Cell culture
The Raji cells were purchased from American Type Culture Collection (ATCC no. CCL-86). The cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (Gibco) and antibiotics (penicillin 100 U/mL, and streptomycin 100 μg/mL). The cells were cultured in 5% CO2 at 37 °C and passaged every 2 days. The cells in logarithmic growth phase were used for in-vivo studies.
Biodistribution studies
All animal experiments were approved by the 4th Local Animal Ethics Committee in Warsaw (authorization number 34/2013), and were carried out in accordance with the national legislation regarding laboratory animals protection and the principles of good laboratory practice.
The human lymphoma cell lines (Raji) was used for xenografts. Male Rj: NMRI-Foxn1nu/Foxn1nu mice (from Janvier Lab. France) were subcutaneously grafted with 106 cells (cell suspension in 200 μL of Matrixgel™ Basement Membrane Matrix (BD Sciences) on the left or right shoulder, under anesthesia with 2% isoflurane. The experiments were performed 2–3 weeks after implantation, when a tumour reached a volume of approximate 100–300 mm3. In a typical experiment, a groups of 3–4 mice were injected (tail vein) with 10 μg (6 MBq) of 90Y- and 177Lu- DOTA-Rituximab in 100 µL each. The animals were euthanized by overdose of isoflurane at 4, 24, 48, and 72 h post-injection (p.i.). Samples of blood and selected organs were collected, weighed and their radioactivity was measured by the NaI gamma counter supplied with adapter for the whole body measurement in case of luthetium-177. The organ uptake values were expressed as the percent of injected dose per organ (%ID) and per gram of tissue (%ID/g). Additionally, the mice injected with 90Y-DOTA-(SCN)-Rituximab were optically imaged at different time points using the PhotonIMAGERTM System (Biospace LAB), based on the detection of Cerenkov emission.
Data Analysis
A two-way analysis of variance (ANOVA) performed with GraphPad Prism 5 for Windows, was applied for comparison of radioactive DOTA-Rituximab concentration in the blood, urine and selected organs in the different treatment groups from the biodistribution study. The Post-hoc Bonferroni analysis was applied for determination of the statistically significant relationships among the treatment groups. A p-value of < 0.05 determined statistical significance. All results were expressed as mean ± s.d.
Results and Discussion
Conjugation of chelating agents to anti-CD20 and radiolabelling
The two DOTA derivatives were conjugated to Rituximab with an initial molar ratio 10:1 for SCN-DOTA and 100:1 for NHS-DOTA, which resulted in approximately 5 and 18 of the chelator molecules per the mAb, respectively. The DOTA-Rituximab conjugates were radiolabeled with 177Lu and 90Y with radiochemical yields ranging from 98% to 100% and being prepared with specific activity from 100 to 900 MBq/mg. The influence of specific activity on radiochemical purity is presented in tables 1 and 2 (where the first value is the percent of a sum of aggregates and monomer, while the second one, presented in brackets, corresponds to the percentage of monomer). For both radioimmunoconjugates, optimal specific activities as high as 600 MBq/mg have been reached under the same reaction conditions and the radiochemical yield ranging from 98.7% to 99.0% and from 98.8% to 99.1% at 1 h for 177Lu-DOTA-Rituximab and 90Y-DOTA-Rituximab respectively. The radioimmunoconjugates were stable in 0.9% NaCl and human blood serum for at least 48 h.
| Specific activity [MBq/mg] | Radiochemical purity [%] at time (n = 2-3) | |||||||
|---|---|---|---|---|---|---|---|---|
| 177Lu-DOTA(SCN)-Rituximab | 177Lu-DOTA(NHS)-Rituximab | |||||||
| 1 h | 24 h | 48 h | 72 h | 1 h | 24 h | 48 h | 72 h | |
| 100 - 300 | 99.7 ± 0.4 | 98.7 ± 0.6 | 96.4 ± 0.6 | 95.4 ± 2.9 | 98.2 ± 0.4 | 95.9 ± 1.9 | 93.8 ± 2.8 | 94.9 ± 2.5 |
| 301 - 600 | 99.0 ± 0.9 | 96.6 ± 2.1 | 90.5 ± 4.4 | 85.4 ± 4.4 | 99.2 ± 1.3 | 95.6 ± 3.8 | 95.5 ± 2.5 | 94.4 ± 3.4 |
| 601 - 900 | 99.0 ± 0.2 | 97.2 ± 1.8 | 95.1 ± 0.6 | 86.1 ± 1.8 | 98.7 ± 0.1 | 95.0 ± 0.5 | 92.5 ±1.1 | 91.9 ± 0.8 |
| Specific activity [MBq/mg] | Radiochemical purity [%] at time (n = 2-3) | |||||||
|---|---|---|---|---|---|---|---|---|
| 90Y-DOTA(SCN)-Rituximab | 90Y-DOTA(NHS)-Rituximab | |||||||
| 1 h | 24 h | 48 h | 72 h | 1 h | 24 h | 48 h | 72 h | |
| 100 - 300 | 98.9 ± 1.9 | 96.0 ± 2.2 | 92.5 ± 4.3 | 84.1 ± 2.9 | 98.3 ± 1.0 | 97.3 ± 0.1 | 93.3 ± 4.2 | 89.7 ± 2.5 |
| 301 - 600 | 96.6 ± 1.8 | 89.8 ± 4.3 | 90.3 ± 9.5 | 75.4 ± 16.7 | 96.7 ± 1.7 | 93.3 ± 2.8 | 90.0 ± 7.4 | 85.2 ± 9.3 |
| 601 - 900 | 99.1 ± 0.1 | 89.5 ± 5.4 | 84.5 ± 0.6 | 82.4 ± 1.8 | 98.8 ± 1.2 | 94.2 ± 3.0 | 89.3 ±0.1 | 87.0 ± 2.8 |
Biodistribution in the tumour-bearing mice
The results of the tissue distribution of 177Lu-DOTA-(SCN)–Rituximab and 177Lu-DOTA (NHS)–Rituximab conjugates are presented in table 3. The tumour uptake reached a value of 7.3 ± 1.7 %ID/g at 24 h for 177Lu-DOTA -(SCN)-Rituximab and increased to 9.3 ± 1.0 at 72 h p.i.v. In contrast, the tumour accumulation of 177Lu-DOTA-(NHS)-Rituximab reached the maximum value of 7.3 ± 1.7 %ID/g at 24 h and appears to be constant up to 72 h (6.9 ± 0.7 %ID/g). A relatively high accumulation of radioactivity was found in the blood at 4 h p.i.v. (20.1 ± 4.7 and 20.8 ± 1.0 for DOTA-(SCN) and DOTA-(NHS) respectively). However, the conjugates were quickly cleared from the blood stream and the radioactivity decreased to 7.0 ± 0.7 and 6.0 ± 0.9 at 48 h.
The highest uptake was observed for blood-rich organs like spleen and liver. In the spleen 23.7 ± 1.7 %ID/g of 177Lu-DOTA-(SCN)-Rituximab at 4 h was found, which then increased to 27.9 ± 5.3 %ID/g at 48 h p.i.v. Similar results were obtained for 177Lu-DOTA-(NHS)-Rituximab: 18.7 ± 3.1 and 27.4 ± 1.8 %ID/g at 4 and 72 h, respectively.
| 177Lu-DOTA(SCN)-Rituximab | 177Lu-DOTA(NHS)-Rituximab | |||||||
|---|---|---|---|---|---|---|---|---|
| 4 h | 24 h | 48 h | 72 h | 4 h | 24 h | 48 h | 72 h | |
| Blood | 20.1 ± 4.7 | 8.9 ± 3.2 | 7.0 ± 0.7 | 10.7 ± 1.9 | 20.8 ± 1.0 | 13.0 ± 1.7 | 6.0 ± 0.9 | 8.3 ± 1.8 |
| Lung | 9.2 ± 1.1 | 4.6 ± 1.1 | 2.8 ± 0.6 | 5.0 ± 0.3 | 9.8 ± 0.3 | 6.0 ± 0.2 | 3.4 ± 0.7 | 4.2 ± 0.8 |
| Liver | 17.0 ± 1.2 | 10.8 ± 2.0 | 8.3 ± 0.7 | 9.4 ± 1.3 | 15.8 ± 1.0 | 10.2 ± 1.7 | 7.4 ± 1.2 | 8.2 ± 0.8 |
| Spleen | 23.7 ± 1.7 | 19.2 ± 10.8 | 27.9 ± 5.3 | 17.6 ± 10.0 | 18.7 ± 3.1 | 28.7 ± 4.1 | 24.9 ± 2.9 | 27.4 ± 1.8 |
| Kidneys | 6.3 ± 1.9 | 4.4 ± 0.7 | 3.4 ± 0.5 | 4.5 ± 1.7 | 7.8 ±1.9 | 4.6 ± 1.1 | 3.7 ± 0.8 | 3.3 ± 0.5 |
| Bone | 8.6 ± 0.3 | 4.9 ± 0.6 | 5.9 ± 0.5 | 4.0 ± 1.0 | 4.9 ± 0.5 | 6.3 ± 2.3 | 5.6 ± 0.5 | 6.9 ± 2.0 |
| Muscle | 1.3 ± 0.6 | 0.9 ± 0.1 | 0.5 ± 0.1 | 1.0 ± 0.0 | 1.7 ± 0.3 | 1.2 ± 0.2 | 0.9 ± 0.1 | 0.9 ± 0.1 |
| Tumour | 1.9 ± 0.2 | 7.4 ± 1.4 | 5.8 ± 1.0 | 9.3 ± 1.0 | 2.7 ± 0.4 | 7.3 ± 1.7 | 6.9 ± 0.6 | 6.9 ± 0.7 |
| Urine [%ID] | 6.0 ± 1.1 | 14.8 ± 0.2 | 25.4 ± 1.7 | 27.8 ± 0.6 | 1.8 ± 0.4 | 8.8 ± 2.0 | 19.2 ± 1.5 | 28.3 ± 4.7 |
At the established time points, when 177Lu-DOTA-(SCN)-Rituximab was administered, the %ID urinary excretion was 6.0 ± 1.1, 14.8 ± 0.2, 25.4 ± 1.7 and 27.8 ± 0.6 while for 177Lu-DOTA (NHS)-Rituximab it was 1.8 ± 0.4, 8.8±2.0, 19.2 ± 1.5 and 28.3 ± 4.7 % ID at 4, 24, 48 and 72 h, respectively. The percentage of excreted radioactivity increased with time post injection for both chelators but the only statistically significant difference (P < 0.0001) was observed at 48 h.
Differences in bone uptake between DOTA-(SCN) and DOTA-(NHS)-Rituximab were observed at 4 h p.i. (8.6 ± 0.3 for 177Lu-DOTA-(SCN)-Rituximab and 4.9±0.5 for 177Lu-DOTA-(NHS)-Rituximab). However, in case of 177Lu-DOTA-(SCN)-Rituximab the radioactivity in bone significantly decreased to 4.0 ± 1.0 % ID/g at 72 h post injection and it was lower compared to the DOTA-(NHS) radioimmunoconjugates 6.9 ± 2.0 %ID/g.
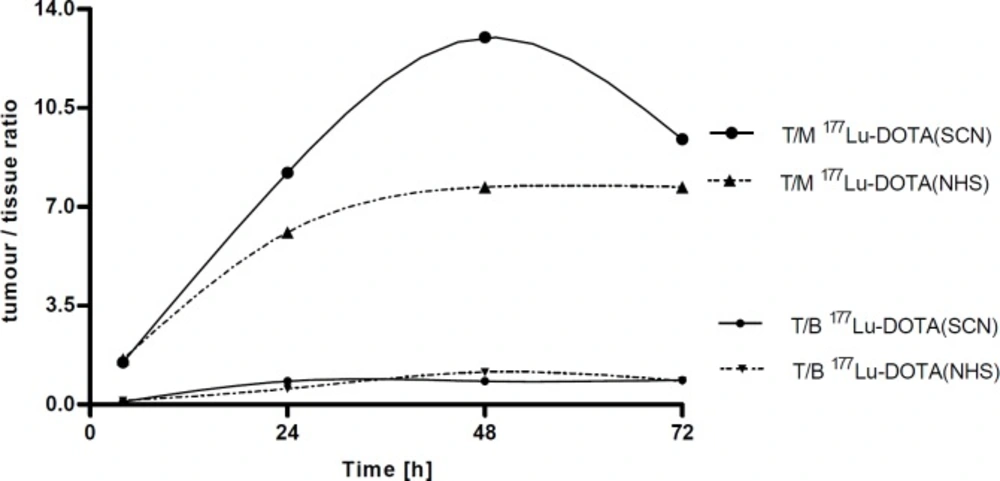
The177Lu-DOTA-(SCN)-Rituximab conjugate demonstrated a higher tumour to muscle ratio (T/M), i.e. 1.5, 8.21, 13.0 and 9.4 at 4, 24, 48 and 72 h, respectively. However, it was not statistically significant (P > 0.05) compared to T/M observed for 177Lu-DOTA-(NHS)-Rituximab (1.6, 6.1, 7.7 and 7.7 at 4, 24, 48 and 72 h, respectively; Figure 1).
It was noted that for 177Lu-DOTA-(SCN)-Rituximab the T/nT tissue ratios reached maximum values of 0.8, 0.7, and 0.4 for blood, liver, and spleen, respectively at 24 h p.i. The tumour to bone ratio peaked to 2.3 at 72 h. The 177Lu-DOTA-(NHS)-Rituximab conjugate showed a little higher T/nT ratios for the blood-rich organs than for DOTA-(SCN) chelator but not statistically significant.
In-vivoCerenkov imaging of 90Y-DOTA-Rituximab
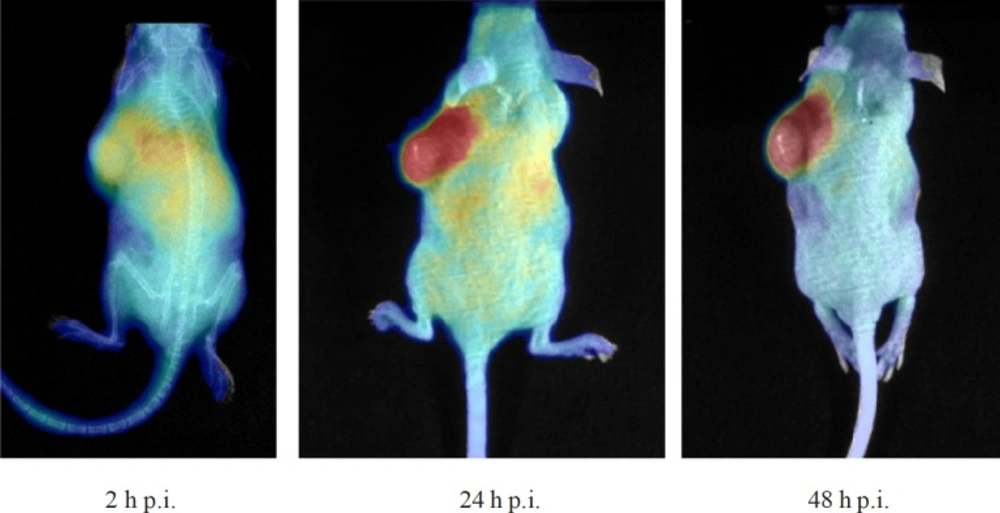
The mice injected with 90Y-DOTA (SCN)-Rituximab were optically imaged at 2, 24 and 48 h p.i.v., (Figure 2). It could be clearly seen that radioimmunoconjugates were accumulated in the liver as well as in the spleen at 2 h p.i.v. The highest accumulation in tumour tissue was excellently visible in the optical images at 24 h p.i.v. The next 24 h observation showed decrease at non-target organs and retention of activity in the tumour. These observations in optical imaging corresponds with those from the biodistribution measurements.
Conclusion
Previously we reported the development of a freeze-dried kit formulation for DOTA-(SCN)-Rituximab radiolabelling with lutetium-177 and yttrium-90 (1), which enabled convenient preparation of the radioactive solution for injection with yields higher than 98%. We also investigated bioconjugate’s stability in serum and 0.9% NaCl up to 48 h. The developed DOTA-(NHS)-Rituximab kit could be labelled straightforward with the radiochemical yield of more than 98% and additionally with less aggregation of the mAb then DOTA-(SCN)-Rituximab.
The biodistribution study in xenografted mice with 177Lu-DOTA-Rituximab showed that the main route of elimination was hepatobiliary tract and no statistically significant differences between the DOTA-(SCN) and DOTA-(NHS) conjugates were observed. The in-vivo stability of described radiolabelled complexes was confirmed by a low accumulation in bone, considering a high affinity of free 177Lu to bone tissue (25, 26). The increased uptake of 177Lu-DOTA-Rituximab over time was observed in tumour. We also observed a stable but low tumour/blood ratio c.a. 1 (Figure 1) which should be > 2 as described by Reilly (27). The bone/blood ratio increased with time (from 0.2 to 0.8 at 4 and 72 h p.i.v., respectively) for 177Lu-DOTA (NHS)-Rituximab but did not increase since 24 h p.i.v., for 177Lu-DOTA-(SCN)-Rituximab. It suggested a higher stability of the metal-ligand complexes of 177Lu-DOTA-(SCN)-Rituximab.
Optical imaging systems can be used for imaging in pre-clinical models. In-vivo Cerenkov imaging of 90Y-DOTA-Rituximab in tumour bearing mice confirmed accumulation of radioactivity in tumour.