Introduction
Breast cancer is the most common cancer type and the main cause of the cancer related deaths in women (1). Oxidative stress that causes the generation of mutagenesis has a key role in cancer development. Loss of cellular redox homeostasis and elevated levels of oxygen free radicals can be tumorigenic (2). Free radicals can damage DNA, proteins, cell membrane, and mitochondria, and they can cause breast cancer. Imbalance between pro-oxidants and anti-oxidants has an important role in breast carcinogenesis (3). Several enzymes such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) have a significant role in antioxidative defense (4). Many studies have shown that antioxidants inhibit free radical oxidative damages (5). Many dietary products such as phytochemicals, dietary fiber, specific foods micronutrients and food constituents have been studied for breast cancer treatment (6). The most commonly used herbs in complementary-alternative medicine therapies is Urtica dioica L (UD) in Turkey (7). UD includes the compounds such as steroids, terpenoids, phenylpropanoids, lignans, coumarins, polysaccharides, lectins and flavonols (8). The phenolic compounds of UD have antioxidative action. The phenolic compounds stabilize lipid peroxidation. Polyphenolic compounds in UD may have inhibitory effects on mutagenesis and carcinogenesis (9, 10). Urtica dioica agglutinin is a lectin that has immunostimulating activity, (8, 11) and supposed to interact with the epidermal growth factor receptor (11, 12). Also, significant antibacterial effects of the extract of UD have been shown (13).
Nowadays, the studies about the use of alternative medicine in the treatment of cancer are quite popular. It was found that UD inhibited the growth of prostate cancer, which is a hormone-dependent cancer. There are no in-vivo studies in the literature about the antioxidant, and antitumor effects of UD in breast cancer until now. For this reason, present study was aimed to investigate the effects of UD on lipid peroxidation, and antioxidant enzyme activities in NMU-induced rat mammary tumorigenesis.
Experimental
Animals
The rats in the study housed in a polyethylene cage which contained shavings feed and water containers. Feed and water were given ad libitum. Temperature (22 ± 2 ºC), light (12 h cycle) and humidity (50%) in the animal room were controlled. NMU (Sigma Chemical Company, Munich Germany) was used as direct carcinogens to form breast cancer. The experiments were performed under the Animals (scientific procedures) Act of 1986 and conform to the National Institutes of Health guidelines for the use of experimental animals. The experimental protocol was approved by the Local (Fırat University) Ethics Committee for Animal Experiments (protocol number 1015).
Experimental protocol
Sixty female Wistar rats (21 days old) were used in this study. The rats were divided into four 4 groups; Group 1 (untreated group, n=15); the rats were fed with control food. Group 2 (NMU group, n=15); NMU was given 50 mg/kg i.p. to rats, and they were fed with control food. In this group, two rats died. Therefore, the study was carried out with 13 rats. Group 3 (NMU +UD, n=15); the time and dose of NMU were similar to the group 2. The rats were fed with 50 g/kg food containing UD until the end of the experiment. Also 50g/L UD was boiled. The rats were fed with water containing UD for 5.5 months. Group 4 (UD, n=15); rats were fed with food and water containing UD as similar to the group 3.
Preparation of plant and chemical material
NMU (50 mg/kg) was once administered by intraperitoneal injection (14). A few drops of 3% acetic acid were used for dissolving NMU. It was diluted with distilled water for preparing a stock solution of 10 mg NMU/ML. NMU was injected within 2 h after preparation (15). UD was provided by local plant stores. Dried UD was powdered by a blender. After the mixture of 20gr powdered UD and 400 ML water was boiled for 15 minutes, it was filtered (10). This water containing UD was given to the rats during 5.5 months. 50 g/kg powdered UD was added into the food of rats. Then, the food was kneaded with water, and shaped by hand. The rats were fed with this food for 5.5 months.
Sample collection and preparation
During six weeks after NMU injection, rats were weekly palpated to determine the mammary tumors. At the end of the study, the rats were anesthetized by the chloral hydrate (Sigma, St. Louis, MO, USA), and decapitated. The mammary tumors, mammary tissue and blood samples were taken at the end of the 22nd week of the experiment. Mammary tumors were sent to the pathology laboratory in 10 % formalin for histopathological examination.
Biochemical analysis
The levels of plasma MDA were measured by the thiobarbituric acid (TBA) method which was modified using the methods of Satoh (16) and Yagi (17). Lipid peroxidation was measured as the production of MDA which in combination with TBA forms a pink chromogen compound whose absorbance at 532 nm was measured. SOD (EC 1.15.1.1) activity was measured using (Randox Ltd, CruMLin, Co Antrim, UK) by colorimetric method. This method uses xanthine and xanthine oxidase to generate superoxide radicals, which react with 2-(4-iodophenyl)-3-(4-nitrophenol)-5 phenyltetrazolium chloride (p-iodonitrozolium violet; I.N.T.) to form a red formazan dye. A spectrophotometer was used for the measurement of SOD activity in 37 °C and 505 nm. The superoxide dismutase activity was measured by the degree of inhibition of the reaction. 5 ML sample of heparinized blood was centrifuged for 10 min in 3.000 r.p.m., and plasma was removed. The red cells were washed with 0.9 % sodium chloride, and cold water was added. Then, it was stored in 4°C for 15 min. At least, dilution of the solution was made by adding a phosphate buffer solution (pH=7.0). CAT (EC 1.11.1.6) activity was measured by the Aebi method (18). The principle of the assay is determined by monitoring the disappearance of H2O2 as spectrophotometrically at 240 nm. Measurement of GSH-Px activity was performed by the Paglia & Valentine method (19) using the Ransel Kit of the Randox Company by the Olympus AU600 (Olympus Optical Co. Ltd., Tokyo, Japan) autoanalyser.
Histopathological examination
Mammary gland tissue of all rats was removed after decapitation. Tissue samples were sent to the pathology laboratory in 10% formalin for histopathological examination. 4 μm thick sections from paraffin-embedded tissues staining with Hematoxylin-eosin (H.E) were examined under a light microscope (Olympus BX50).
Statistics
Statistical analysis was conducted by using the SPSS (Statistical Program for Social Sciences) software package. Comparisons between groups were performed by one-way analysis of variance (ANOVA) followed by the Tukey HSD test. In addition, The Fisher's exact test was used for categorical comparisons in different groups. P<0.05 was considered statistically significant.
Results
Effect of UD on plasma MDA level, and, SOD, CAT, GPx enzyme activities
Plasma MDA levels and erythrocyte antioxidant enzyme activities were shown in Table 1. There was a significant increase in plasma MDA levels of group 2 compared with group 1 and 4 (p<0.05, p<0.01 respectively). Group 3 plasma MDA levels found similar to the plasma levels of group 1. Group 3 MDA levels showed decrease when compared with group 2 but, there was no statistical significance (p>005). The SOD activity in group 3 was significantly lower than other groups (for all, p <0.0001). Group 4 (UD group) showed a slight increase in SOD activity than the control group (p>0.05). GSH-Px levels did not show the difference between groups. Group 4 (UD group) showed a slight increase in GSH-Px activity than the control group (p>0.05). Catalase activities of Group 2 and group 3 were significantly lower than group 4 (p<0.01 and p<0.05, respectively).
p <0,01, compared with group 4
p <0.05, compared with group 1
p<0.0001, compared with group 1
p<0.0001, compared with group 2
p<0.0001 compared with group 4
p<0.05, compared with group 2
p<0.01, compared with group 3
Effect of UD on the number of animals with tumors
The number of animals with palpable tumors was 6 (46.15%) in group 2 and 2 (13.3%) in group 3 at the end of the 22nd week. Although MNU+ UD group had lower palpable tumor number, the difference was not statistically significant (p=0.096) (Table 2).
Histopathological changes in mammary gland tissue
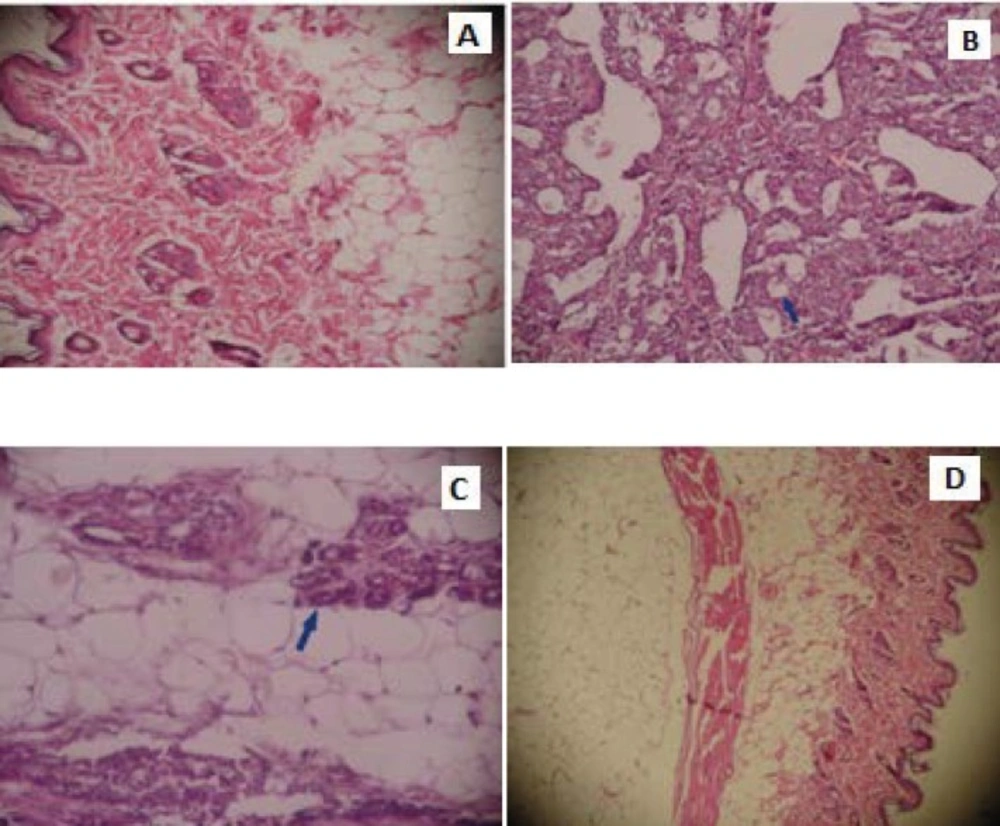
The untreated group (Group 1) in histopathological examination showed normal ductular and alveolar structure of mammary gland tissue and epithelial cells with uniform appearance (Figure 1A). In Group 2, irregularly shaped glands tented to coalesce in the tumoral areas. Tumor cells that compose the core of pleomorphic hyperchromatic and prominent nucleoli were found from place to place. In this group, invasion was seen in several mammary gland tissue (Figure 1B). The animals treated with UD (Group 3) showed mild ductular proliferation with focal epithelial hyperplasia (Figure 1C). The animals given only UD did not show an observable distinct change in compared with the control (Figure 1D).
Histopathological examination of breast tissue in control group and experimental groups. The tissue of untreated rat (A) (HEx200), The breast tissue of the rat induced with the NMU (B) (HEx200), The breast tissue of the rat fed with UD, induced with the NMU (C) (HEx200), The breast tissue of the rat fed with UD (D) (HEx200
Discussion
According to current knowledge, this is the first study showing the changes on antioxidant enzymes using UD in rats generated mammary tumors. It is known that increased oxidative stress plays a major role in the pathogenesis of solid tumors (20). Increased cell proliferation can bring an increase in production of ROS and lipid peroxidation. Also, the products of lipid peroxidation can cause cell proliferation by cell signalling (21). In this study, plasma lipid peroxidation levels in the breast cancer group when compared to the control groups were increased. Although UD has decreased lipid peroxidation levels in group 3 than group 2, the difference was not statistically significant. More interestingly, the MDA levels of the control group fed with UD was found lower than the control group fed with control food. The phenolic compounds of UD may have reduced the MDA levels. In a study carried out by Kanter et al. (22) used the UD and Nigella Sativa in the CCl4-treated rats. The authors showed increased antioxidant defence system activity and decreased lipid peroxidation and liver enzymes in these rats. Cetınus et al. (9) showed that the extract of the UD inhibited the lipid peroxidation by more than %50.
In the present study, a significant decrease in SOD activity of group 3 compared to other groups was seen. SOD can be defined as a family of ubiquitous antioxidant metalloproteinases that catalyze the conversion of superoxide anion radicals to hydrogen peroxide and molecular oxygen (23). In a study the water extract of UD was used. The authors suggested that UD had strong hydrogen donating ability, a metal chelating ability, and effectiveness as scavengers of hydrogen peroxide, the superoxide anion and free radicals (10). We have thought that SOD gene expression may have been suppressed due to the ability of scavenging of superoxide anion radicals of UD.
In our study, the high CAT activities in the group fed with UD showed that UD had a powerful antioxidant effect. The constituents of UD may be the reason of increased catalase activity in the group fed with UD. A study reported that the water extract of UD showed more effective antioxidant activity than the -tocopherol on peroxidation of linoleic acid (10). In this study, a significant difference of GPx activity between the groups was not found. A significant increase in the activity of GPx, which is the first step of enzyme defense against H2O2 and other hydroperoxides, has been reported in tumors (24). When the SOD enzyme activity increases in the tumor tissue, GSH-Px and catalase enzyme activities increase for supporting detoxification of H2O2. In this study, SOD activity did not increase in all groups. Unchanged GPx activities may be responsible for this result. The group fed with UD showed a slight increase in SOD and GPx activities while the group showed a significant increase in catalase activity.
Although the percentage of cancer in the rats fed with UD was lower than the rats fed without UD, there was no statistical difference between the groups with cancer. Although the tumor formation was seen in only two animals in third and fourth months in MNU+ UD group, there was not any tumor formation in the fifth month. However, in several studies, it has been shown that UD inhibit the prostate cancer with various mechanisms (25, 26). In the literature, there were not any in-vivo studies about the effects of UD on mammary cancer. Güler et al. (27) demonstrated that UD had a key role in the prevention of the growth of cells by inhibiting the adenosine deaminase in prostate cancer. Furthermore, it was shown that the polysaccharide fraction of UD inhibited the growth of prostate epithelial cells (25). In an in-vitro study carried out Fattahi et al. (26) reported that the aqueous extract of the leaf of Urtica dioica had the antiproliferative effect in a breast cancer cell line. In this present study, it has been thought that specific constituents or combinations of phytochemicals in UD may have an effect blocking the initial stage in rats with mammary tumor.
In this study, when the histological mammary tissue sections examined, progression to invasion in the mammary gland tissue was observed in the group applied the NMU However, the majority of the group given NMU + UD did not show histologically changes or showed mild hyperplasia. Flavonoids, caffeic malic or unknown substances in UD extracts can mediate NF-κB inhibitory effects, which plays an essential role in the development and progression of breast cancer (28, 29). The authors thought that anticancer effect of UD could be occurred through the inhibition of the NF-κB activation cascade (29).
conclusion
In conclusion, we found that UD reduced the lipid peroxidation and caused a significant increase in catalase enzyme activity. Although the percentage of cancer in rats fed with UD was lower than the rats fed without UD, no statistical difference between these two groups was seen. The initiation and progression of mammary cancer may be slow down by a variety mechanisms of UD, including strengthening the immune system, preventing lipid peroxidation and the effects of phenols. Unfortunately, these mechanisms are not clear yet. It will be better to support these findings with clinical studies.