Introduction
The genus Stachys is one of the largest genera in the flowering plant family of Lamiaceae with about three hundred species distributed in Europe, Asia, Africa, Australasia, and North America(1).
Stachys lavandulifolia Vahl is an herbaceous wild plant native to Iran (2) which is used in Iranian folk medicine as a mild sedative tea for reducing anxiety and for treatment of gastrointestinal disorders (3).
Previous study on hydroalcoholic, polyphenolic, and boiled extracts of S. lavandifolia demonstrated their analgesic effect on animal models (4). In another study, antimicrobial and antioxidant activities of the essential oil of S. lavandulifolia were observed (5). Our previous study on S. lavandulifolia hydroalcoholic extract by the elevated plus-maze (EPM) model of anxiety in mice showed anxiolytic effect at therapeutically acceptable doses (6). Further study on petroleum ether, ethyl acetate, butanol and aqueous fractions on spontaneous motor activity and elevated plus-maze behavior in mice showed interesting anxiolytic properties for ethyl acetate fraction (7) and thus, we decided to investigate the main chemical constituents responsible for anxiolytic effects of ethyl acetate extract of S. lavandulifolia.
In previous researches on polar fractions of methanol extract of this plant, lavandulifolioside A, lavandulifolioside B, verbascoside, leucosceptoside A, 5-O-allopyranosyloxy-aucubin together with three phenylethanoid glycosides were reported (8). The essential oil of aerial part of S. lavandulifolia was also analyzed by GC/ MS method and germacrene-D (13.2%), β-phellandrene (12.7%), β-pinene (10.2%), myrcene (9.4%), α-pinene (8.4%) as well as Z-β-ocimene (5.8%) reported as the main components of the essential oil (9).
Experimental
General
NMR spectra were recorded on a Bruker Avance AV 400, using methanol-d4 as solvent. ESI-MS spectra were measured in positive and negative mode on Shimadzu 2010EV LC-MS system (Shimadzu, Japan). Column chromatography runs were performed using Silica gel, 63-200µm (Merck) and polyamide SC6 (Macherey- Nagel, Duren, Germany). HPTLC was performed on silica gel GF254 plates (Merck, Darmstadt, Germany). Plates were developed by Cerium sulphate (1 g in 5% H2SO4) or natural product reagent (1% methanolic diphenyl-boric acid-ethanolamine) and visualized by UV-fluorescent colours at 254 /366 nm UV lamps. Recycle HPLC was done on a modified Waters HPLC apparatus (Waters Assoc., Milford, MA, USA) at 250 nm using silica gel column (YMC-Pack SIL, 250 × 20 mm, YMC Co., Kyoto, Japan).
Plant material
S. lavandulifulia was collected from Shahrekord city in Chaharmahal va Bakhtiari Province in the west of Iran. The plant was identified by the Department of Biology, Faculty of Science, University of Isfahan and also a voucher specimen (No. 1113) was deposited in the herbarium of the Isfahan Faculty of Pharmacy, Isfahan University of Medical Sciences, Isfahan, Iran.
Extraction and isolation
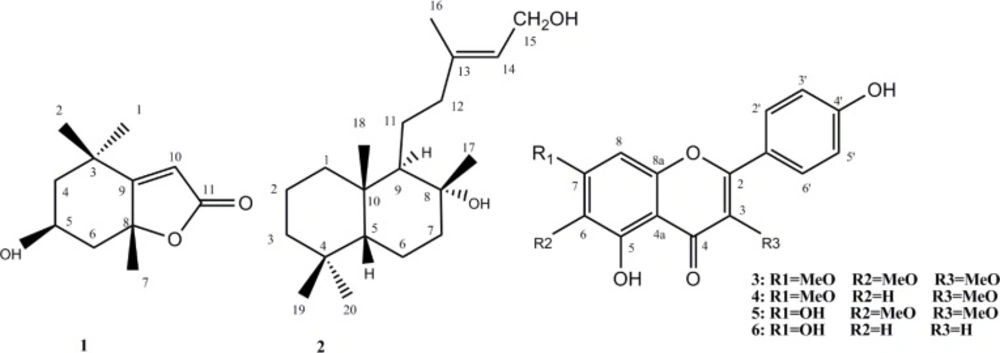
Following with our previous study which ethyl acetate fraction of S. lavadulifolia showed anxiolytic effect based on spontaneous motor activity and elevated plus-maze behavior in Syrian mice (7), in the same order ethyl acetate extract of the plant (5 kg) was obtained. Filtration and vacuum evaporation resulted in a green mass (254 g). After partitioning between hexane and methanol in separatory funnel, lower defatted methanol fraction (56 g) was concentrated and column chromatographed on silica gel (63-200 µm, 400 g) using hexane/acetone, with increasing polarity (5→50 %) to afford five fractions (F1-F5). The fraction of F2 which was eluted by hexane: acetone (9:1) was applied on HPLC using chloroform: methanol (92:8) as solvent and yielded F2c2 (1) as a pure iridoid. F3 eluted by hexane: acetone (8:2) precipitated as yellowish crystals which was more purified by recycle HPLC using chloroform: methanol (90:10) to yield F3b1 (2) and F3b2 (4) as a labdane diterpene and a methoxylated flavonoid, respectively. Finally, F4 eluted with hexane: acetone (7:3) and F5 eluted with hexane: acetone (5:5) were applied on recycle HPLC using chloroform: methanol (85:15) and F4f2b (3), F5c2a (5), and F5e (6) with flavonoid structure were isolated.
Compound 1
Amorphous white powder, MW (g/mol): 196; yield: 0.00016%; 1H-NMR (CDCI3, 400 MHz): δH 5.71(s, 1H, H-10), 4.24 (m, 1H, H-5), 2.45 (1H, bd, J=14.1, H-4b), 1.93 (1H, bd, J=14.1, H-4a), 1.80 (s, 3H, H-7), 1.52 (1H, m, H-6b), 1.49 (s, 3H, H-1), 1.35(1H, m, H-6a), 1.30 (s, 3H, H-2). 13C-NMR data (CDCl3, 100 MHz): 30.6 (C-1), 26.9 (C-2), 35.9 (C-3), 47.3 (C-4), 66.8 (C-5), 45.6 (C-6), 26.5 (C-7), 86.8 (C-8), 173.8 (C-9), 112.9 (C-10), 182.6 (C-11). EIMS m/z 196 [M], 178, 163, 152, 140, 125, 111, 95, 81, 69, 57.
Compound 2
Amorphous white powder, MW (g/mol): 308; yield: 0.00015%; 1H-NMR (CDCI3, 400 MHz): δH 5.38 (t, 1H, J=6.8Hz, H-14), 4.09 (d, 2H, J=6.8Hz, H-15), 1.70 (s, 3H, H-16), 1.14 (s, 3H, H-17), 0.90 (s, 3H, H-18), 0.86 (s, 3H, H-19), 0.84 (s, 3H, H-20). 13C-NMR data (CDCl3, 100 MHz): 41.2 (C-1), 19.5 (C-2), 44.3 (C-3), 34.2 (C-4), 57.5 (C-5), 21.5 (C-6), 43.2 (C-7), 74.9 (C-8), 62.5 (C-9), 40.4 (C-10), 25.2 (C-11), 45.0 (C-12), 140.9 (C-13), 124.1 (C-14), 59.4 (C-15), 16.4 (C-16), 33.9 (C-17), 16.1 (C-18), 21.9 (C-19), 23.8 (C-20). Positive ESIMS m/z 331 [M+Na]+, 309 [M+H] +, 306, 290, 275.
Compound 3
Amorphous yellow powder, MW (g/mol): 344; yield: 0.0022%; 1H-NMR (CDCI3, 400 MHz): δH 8.05 (d, 2H, J=8.8Hz, H-2', H-6'), 6.95 (d, 2H, J=8.8Hz, H-3', H-5'), 6.79 (s, 1H, H-8), 3.99 (s, 3H, OMe), 3.86 (s, 3H, OMe), 3.82 (s, 3H, OMe). 13C-NMR data (CDCl3, 100 MHz): 158.6 (C-2), 139.5 (C-3),180.3 (C-4), 107.3 (C-4a), 154.0 (C-5), 133.4 (C-6), 161.9 (C-7), 92.1 (C-8), 153.4 (C-8a), 122.5 (C-1'), 131.5 (C-2'), 116.6 (C-3'), 160.6 (C-4'), 116.6 (C-5'), 131.5 (C-6'), 60.6 (3-OMe), 61.1 (6-OMe), 57.0 (7-OMe). EIMS m/z 344 [M], 329, 207, 197, 181, 167, 158, 149, 131, 121, 93, 69, 57.
Compound 4
Amorphous yellow powder, MW (g/mol): 314; yield: 0.001%; 1H-NMR (CDCI3, 400 MHz): δH 8.05 (d, 2H, J=8.8Hz, H-2', H-6'), 6.95 (d, 2H, J=9.2Hz, H-3', H-5'), 6.66 (d, 1H, J=2.4 Hz, H-8), 6.37 (d, 1H, J=2.4 Hz, H-6), 3.91 (s, 3H, OMe), 3.81 (s, 3H, OMe). 13C-NMR data (CDCl3, 100 MHz): 158.0 (C-2), 138.2 (C-3), 184.1 (C-4), 105.0 (C-4a), 154.3 (C-5), 98.8 (C-6), 161.4 (C-7), 93.0 (C-8), 154.3 (C-8a), 122.5 (C-1'), 131.2 (C-2'), 116.4 (C-3'), 158.2 (C-4'), 116.4 (C-5'), 131.2 (C-6'), 60.5 (3-OMe), 56.3 (7-OMe). Negative ESIMS m/z 313 [M-1]-, 298, 269, 255, 227, 187, 166, 154, 136.
Compound 5
Amorphous yellow powder, MW (g/mol): 330; yield: 0.0035%; 1H-NMR (CDCI3, 400 MHz): δH 7.89 (d, 2H, J=8.8Hz, H-2', H-6'), 6.83 (d, 2H, J=8.8Hz, H-3', H-5'), 6.41 (s, 1H, H-8), 3.78 (s, 3H, OMe), 3.67 (s, 3H, OMe). 13C-NMR data (CDCl3, 100 MHz): 153.7 (C-2), 139.1 (C-3), 180.3 (C-4), 106.3 (C-4a), 153.8 (C-5), 132.6 (C-6), 158.8 (C-7), 95.0 (C-8), 158.2 (C-8a), 122.5 (C-1'), 131.4 (C-2'), 116.5 (C-3'), 161.8 (C-4'), 116.5 (C-5'), 131.4 (C-6'), 60.6 (3-OMe), 61.0 (6-OMe). Negative ESIMS m/z 329 [M-1]-, 314, 299, 271, 227, 187, 166, 154, 125, 111.
Compound 6
Amorphous yellow powder, MW (g/mol): 269; yield: 0.0065%; 1H-NMR (CDCI3, 400 MHz): δH 7.88 (s, 1H,OH), 7.73 (d, 2H, J=8.8Hz, H-2', H-6'), 6.85 (d, 2H, J=8.8Hz, H-3', H-5'), 6.49 (s, 1H, H-3), 6.37 (d,1H, J=2Hz, H-8), 6.14 (d,1H, J=2Hz, H-6). 13C-NMR data (CDCl3, 100 MHz): 163.8 (C-2), 104.4 (C-3), 183.2 (C-4), 106.5 (C-4a), 163.2 (C-5), 100.5 (C-6), 166.4 (C-7), 95.3 (C-8), 159.0 (C-8a), 122.8 (C-1'), 129.4 (C-2'), 117.3 (C-3'), 163.3 (C-4'), 117.3 (C-5'), 122.8 (C-6'). Negative ESIMS m/z 269 [M-1]-, 257, 227, 195, 181, 173, 155, 129, 110.
Results and discussion
The 13C NMR and 1H-NMR spectra of compound 1 indicated signals of three singlet methyl groups, two methylene, an oximethine, two quaternary carbons which one of them was oxygenated, a tri-substituted olefin bond and a lactone carbonyl with EIMS m/z 196 which resembled with those of a C11 iridoid named loliolide (10). The iridoids and secoiridoids form a large group of plant constituents that are found usually. They are mostly 4, 7-dimethylcyclopentapyran. But in the case of loliolide (C11 iridoid) and its related structures like actinidiolide and aeginetolide their biosynthesis pathway is different from usual C-10 iridoids and secoiridoids. They are generated in variety of plants from photo-oxygenation and degredation of carotenoids (11-12).
Compound 2 was obtained as a white powder. 13C-NMR data in addition to positive ESIMS at m/z 331 [M+Na] + corresponded to the formula C20H36O2. 1H NMR spectrum showed signals attributed to five singlet methyls δH 1.70, 1.14, 0.90, 0.86, and 0.84. Further signals in 1H NMR spectrum at δH 5.38 (t, 1H, J=6.8Hz, H-14) and 4.09 (d, 2H, J=6.8Hz, H-15) indicated the presence of a tri-substituted olefin bond and an external oxymethylen group. The 13C-NMR and DEPT spectra demonstrated twenty carbons comprised of five methyls, eight aliphatic methylens (one oxygenated), two aliphatic methines, two aliphatic quternary carbons (one oxygenated), one olefinic methine, and one quaternery olefinic carbon. Three degrees of unsaturation, and one olefinic bond suggested two rings in the structure. Comparing these characteristic signals with compound 6 isolated from Leonurus heterophyllus by Hung and coworkers (14) and compound 8 isolated from Leonurus heterophyllus by Giang and coworkers (13) and using HMBC spectra determined structure of compound 2 as labda-13-ene-8, 15-diol.
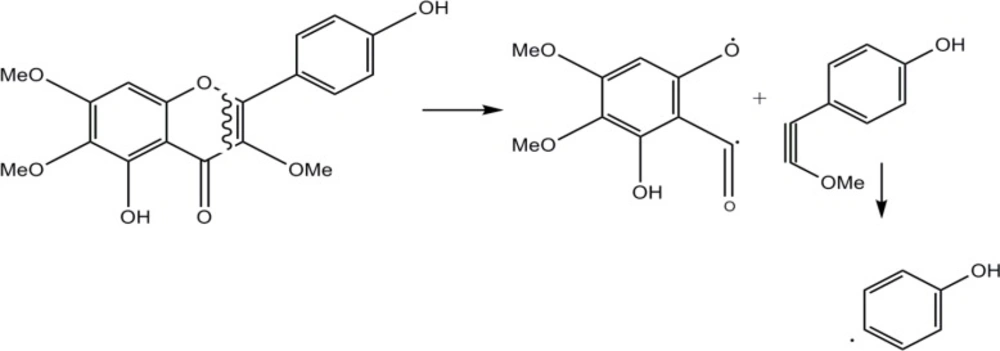
Compound 3 was isolated as a yellowish powder with orange fluorescence reaction to Natural Product reagent (Diphenylboric acid 2-aminoethyl ester 1%). Molecular formula was determined by NMR data and negative EIMS m/z 344 [M] as C18H16O7. Eleven degrees of unsaturation, one carbonyl carbon, and seven olefin bands indicated three rings in the structure. The 1H-NMR spectrum showed a singlet olefin proton at δ 6.79 (s, 1H) described to H-8. Ortho-coupled proton signals( each two protons) at δ 8.05 (d, 2H, J=8.8Hz) and 6.95 (d, 2H, J=8.8Hz) were corresponded to H-2’,6' and H-3', 5'. Three singlet methoxy signals at δH 3.99 (δC 61.1), 3.86 (δC 60.6), 3.82 (δC 57.0) were located on C-3, C-6 and C-7, respectively based on long range HMBC correlation and cleavage pattern of compound in EI-Mass by retro-Diels-Alder mechanism including A (m/z 197), B (m/z 149) and C (m/z 93) ions. All of these data defined the structure of 3 as 3, 6, 7-trimethoxy-4',5-dihydroxyflavone named as penduletin which was in agreement with literature data (15). Figure 2 shows the fragmentation pattern of this compound by cleavage of ring C via retro-Diels-Alder mechanism.
Compound 4 assigned the molecular formula of C17H14O6 based on negative pseudo-molecular ion [M - H]- at m/z 313 and 13C-NMR (BB and DEPT). 1H & 13C-NMR spectral data cleared that compound 4 and 3 have similar structures but differed in lacking methoxy group at C-6. Comparison of the spectral data with literature data determined the structure as 7,3-dimethoxy-4',5-dihydroxyflavone named as Kumatakenin (16). Similarly, NMR spectral data cleared that compounds 5 and 3 resembled each other, except for the 7-O-hydroxyl instead of 7-O-methoxyl group which was confirmed by pseudo-molecular ESI Mass ion [M - H]- at m/z 329. It was defined as 6, 3-dimethoxy-4', 5-dihydroxyflavone (17). Compound 6 was proved to be 4', 5, 7-trihydroxyflavone named as apigenin (17).
Many species of the genus Stachys have been investigated and different kinds of secondary metabolites have been isolated, mainly flavonoids, iridoids and terpenoids from the aerial parts and the roots (18). In this investigation, an iridioid, a labdane type diterpene, three methoxylated flavonol derivatives, and a flavone were isolated.
Previous phytochemical studies showed presence of several types of flavonoids in Stachys genus. Some Stachys species are rich in flavonoids, for example 24 flavonoids were identified in S. aegyptica and 9 in S. ionica (19, 20). However in this study, three methoxylated flavonol derivatives, and a flavone were isolated from S.lavandulifolia. Compound 3 with trivial name of kumatakenin, previously was isolated from Ballota hirsuta, Eupatorium illitum, Achillea kotschya, and Baccharis petiolata. The flavonoids from Eupatorium illitum have been presented antiproliferative activity (22). Compound 4 with trivial name of penduletin, was previously isolated from Achillea nobilis, Isocoma tenuisecta and Betola nigra (21). Pendulentin has showed antiproliferative property (23) and strong activity against enterovirus (24). Compound 5, was previously reported from Achillea kotschya, Achillea nobilis, and some other genera of Asteraceae and apigenin is also determind in many Stachys species 21).
The diterpenoids isolated from different species of Stachys are structurally different with labdane, kauran, clerodane, and abietan skeletone. Kauran diterpenoids are present in S. silvatica, S. lanata, and S. sprumeri and also S. recta, neo-clerodan derivatives are found in S. annua, S. rosea, and S. recta. An abietan derivative in S. officinalise and diterpenoids with labdane skeleton were also obtained from S. mucronata, S. plumasa, and S. menthifolia (18, 25). The labdane diterpenoid which is isolated in this study was also previously reported from S.menthifolia, Leonurus heterophyllus, and Cistus creticus (11, 18).