Introduction
According to the world health organization (WHO) report, there were about 219 million cases of malaria in 2010 and an estimated 660,000 deaths (1). Resistance is now common against all classes of antimalarial drugs and poses a growing problem in the malaria treatment, thus the biggest issue all over the world is to combat malaria with safe and effective medications and to avoid the emergence of drug-resistant malaria parasites (2). Of the various antimalarial drugs available, the aminoquinoline chloroquine was the agent of choice for many decades because of its safety, efficacy and affordability.
The quinoline scaffold is prevalent in a variety of pharmacologically active synthethic and natural compounds (3). The quinolines such as chloroquine, mefloquine, amodiaquine, primaquine, and quinine are historically among the most important antimalarial drugs ever used. The drugs from this group mostly act during the blood stages of the parasite’s life cycle but some target the hepatic stages as well (4). The quinolines are known to inhibit the polymerization of heme and prevent disposal of polymers from the food vacuole to the cytoplasm where hemozoin is formed. This leads to intraparasitic accumulation of free heme, which is highly toxic to the parasite (3).
Chloroquine (CQ), a 4-aminoquinoline, was first chemically synthesized in 1934, as a substitute for quinine. Since its discovery, CQ was the best antimalarial drug according to its safety, affordability, and efficacy. Despite this, the emergence and rapid spread of resistance of Plasmodium falciparum to CQ and other related antimalarials has dramatically reduced the therapeutic options (5) and have created an urgent need to discover new antimalarial agents (6). Researchers around the world have synthesized a large number of CQ analogues with the hope to overcome its drug-resistance properties (3, 7-10). In the context of our on-going research, we wished to synthesize novel compounds for screening against P. falciparum and to this end we reported synthesis of novel α-(acyloxy)-α-(quinolin-4-yl) acetamides by a three component reaction between an isocyanide, quinoline-4-carbaldehyde and arenecarboxilic acides (11).
Experimental
Chemicals
All materials and reagents in this study were purchased from Merck (Darmstadt, Germany) and Sigma Aldrich (Steinheim, Germany). RPMI 1640 medium and AlbumaxI prepared from Gibco-Invitrogen (Paisley, Scotland, UK).
Tested compounds
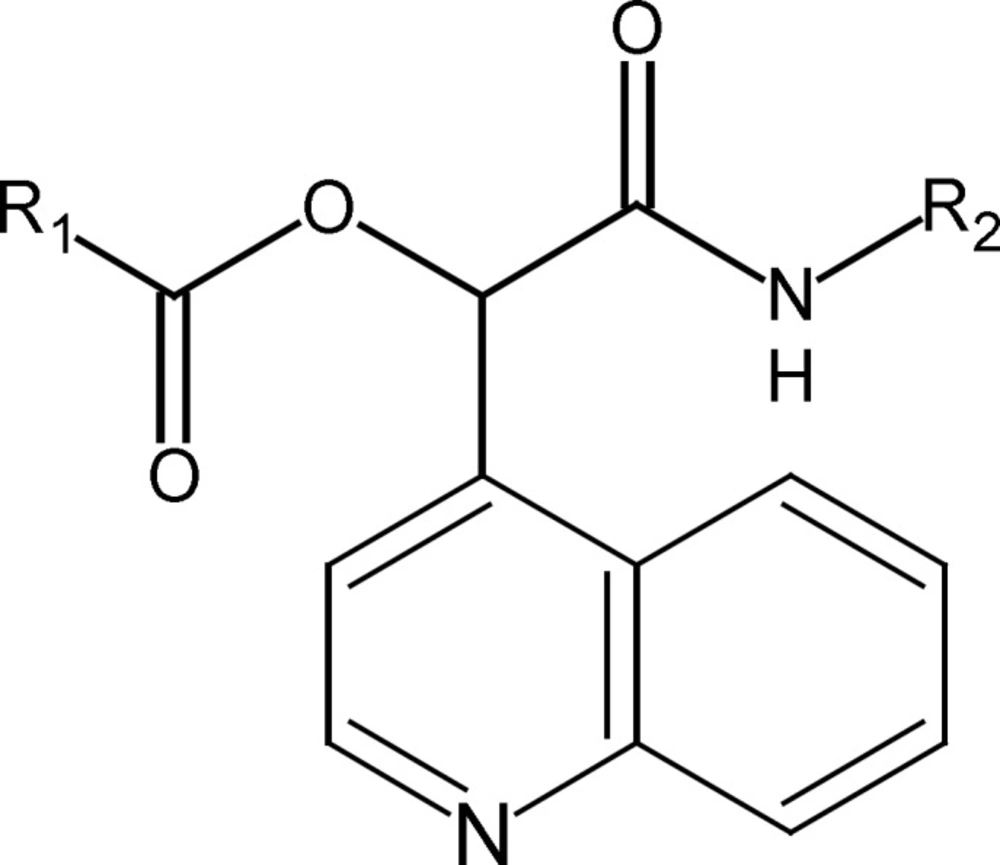
We synthesized previously some novel α-(acyloxy)-α-(quinolin-4-yl) acetamides by a three component reaction between an isocyanide, quinoline-4-carbaldehyde and arenecarboxilic acides (11). The basic structure of α -(acyloxy)-α-(quinolin-4-yl) acetamides derivatives is indicated in Figure 1.
Parasite Culture
The P. falciparum 3D7 chloroquine-sensitive strain used throughout the study. In-vitro culture of P. falciparum was carried out according to the method described by previously (12-14). Briefly, parasites were cultured on human erythrocytes (blood group O+), provided by the Blood Transfusion Organization (Zanjan, Iran), in RPMI 1640 medium completed with 5% of human AB+ serum, 0.3 g/100 mL Albumax I, 25 mM HEPES, 19 mM sodium carbonate and 30 µg/mL gentamicin sulfate at pH 7.2. Type O+ erythrocytes were washed three times with RPMI 1640 and stored at 4 °C.
Parasites were aerated in 25 mL flasks under 3% oxygen, 6% carbon dioxide and 91% nitrogen atmosphere. The medium was changed each day.
In-vitro antimalarial tests
Pure compounds were dissolved in DMSO at concentration of 10 mg/mL and diluted with complete malaria culture medium to reach 1 mg/mL before use. Parasites were synchronized to the ring stage by sorbitol method described previously (15). From 2-fold dilution series (50-0.39 µg/mL) of compounds prepared in assay medium, 20 µL added to each well of 96-well microtiter plates in triplicate. One hundred eighty µL of synchronous P. falciparum culture (1% parasitemia, 2% hematocrit) added to each well reaching a final volume of 200 µL per well. Final DMSO concentration reached to 0.4% that was not toxic to parasite. Plates were incubated at 37 °C for 24 h. Chloroquine at 50% inhibitory concentration (0.7 µM) was used as positive control and parasitized erythrocytes without drug were used as negative control. After 24 h incubation, Giemsa stained thin smears were made and parasitemia was confirmed by the numeration of 1000 erythrocytes per slide. Data acquired by counting the erythrocytes in Giemsa stain were imported in Microsoft Excel spreadsheet and IC50 values were calculated from dose-response curves.
In-vitro cytotoxicity assay
In drug discovery approaches against malaria, one of the important strategies is the safety of active compounds against human. So, the toxicity of active compounds against P. falciparum was assessed on human hepatocellular carcinoma cell line (HepG2) by using MTT (3-[4,5-dimethylthiazol-2-yl] -2,5 diphenyl tetrazolium bromide) assay (16-17). The cells were cultured in RPMI 1640 medium enriched with 10% FBS (Fetal Bovine Serum) and incubated at 37 °C with 5% CO2 and 96% humidity. After several subcultures, cells were distributed in 96-well plates at 4,000 cells in 100 µL of culture medium and incubated for 24 h at the same condition to allow attachment of cells to the bottom of wells. Then culture medium removed and 100 µL of two-fold serially diluted concentrations of drug (400-1.56 µg/mL) added to each well in triplicate. Microtiter plates further incubated for 24 h in the same condition. Culture medium without drug was used as negative control. After the incubation time, the drug containing medium discharged and for evaluation of cell survival, 25 µL of MTT solution (4 mg/mL in PBS) added to each well and plates incubated for 3 h (in same condition). Then 100 µL of DMSO added to each well and plates were gently shaken to dissolve the formed formazan crystals. The absorbance of each well measured at 540 nm using an ELISA plate reader (Infinite M200, Tecan). The GI% (Growth Inhibition percent) was calculated using the formula Growth Inhibition% = 100 – (ODtest - ODcontrol) × 100, where ODtest is the mean absorbance of treated cells and ODcontrol is the mean absorbance of a negative control. The cell survival of control assumed 100% and IC50 values generated from dose-response curves for each cell line.
| Compound | R1 | R2 | P. falciparum IC50 (µM) | HepG2 IC50 (uM) | SI |
|---|---|---|---|---|---|
| 1 | Phenyl | cyclohexyl | 1.511 | 229.93 | 152.17 |
| 2 | 4-tert-butylphenyl | cyclohexyl | 2.635 | 190.6 | 72.33 |
| 3 | 4-methylphenyl | cyclohexyl | 2.781 | 202.02 | 72.64 |
| 4 | 4-chlorophenyl | cyclohexyl | 5.017 | - | - |
| 5 | 3-chlorophenyl | cyclohexyl | 6.413 | - | - |
| 6 | 4-fluorophenyl | cyclohexyl | 7.255 | - | - |
| 7 | 4-bromophenyl | cyclohexyl | 5.792 | - | - |
| 8 | 4-iodophenyl | cyclohexyl | 3.497 | - | - |
| 9 | 4-cyanophenyl | cyclohexyl | 3.645 | - | - |
| 10 | 1-naphthyl | cyclohexyl | 3.7 | - | - |
| 11 | Phenyl | tert-butyl | 4.346 | - | - |
| 12 | 4-tert-butylphenyl | tert-butyl | 3.001 | - | - |
| 13 | 4-chlorophenyl | tert-butyl | 5.849 | - | - |
| 14 | 3-chlorophenyl | tert-butyl | 6.417 | - | - |
| 15 | 4-fluorophenyl | tert-butyl | 5.056 | - | - |
| 16 | 4-bromophenyl | tert-butyl | 5.103 | - | - |
| 17 | 4-cyanophenyl | tert-butyl | 3.085 | - | - |
| 18 | 1-naphthyl | tert-butyl | 6.953 | - | - |
| 19 | Phenyl | 2,6-dimethylphenyl | 4.314 | - | - |
| 20 | 4-tert-butylphenyl | 2,6-dimethylphenyl | 1.373 | 199.07 | 144.98 |
| 21 | 4-chlorophenyl | 2,6-dimethylphenyl | 4.177 | - | - |
| 22 | 1-naphthyl | 2,6-dimethylphenyl | 1.325 | 254.27 | 191.90 |
| CQ | - | - | 0.7 |
Results and Discussion
The antimalarial activity of all compounds was evaluated against P. falciparum 3D7 chloroquine-sensitive strain. The antiplasmodial activities were determined as inhibitory concentrations at 50% parasite survival (IC50) in the strain and are tabulated in Table 1. Compounds 1, 20 and 22 showed significant antiplasmodial activity with IC50 value of 1.511, 1.373 and 1.325 µM, respectively. The IC50 values of these three compounds somewhat is near to CQ as the standard quinoline antimalaral drug. Compounds 2 and 3 also showed moderate activity with IC50 values of 2.635 and 2.781 µM. The rest of compounds did not show noticeable antiplasmodial activity.
The cytotxicity of compounds with IC50 value less than 3 µM assessed on HepG2 cell line. Results of toxicity activity of the tested compounds and selectivity index (SI) are shown in Table 1. The SI is defined as the ratio of the HepG2 toxicity to the antiplasmodial activity and the higher selectivity should offer the potential of safer therapy without adverse effect in human. The 1, 20 and 22 compounds had high selectivity for P. falciparum than HepG2 cell line in comparison with other compounds.
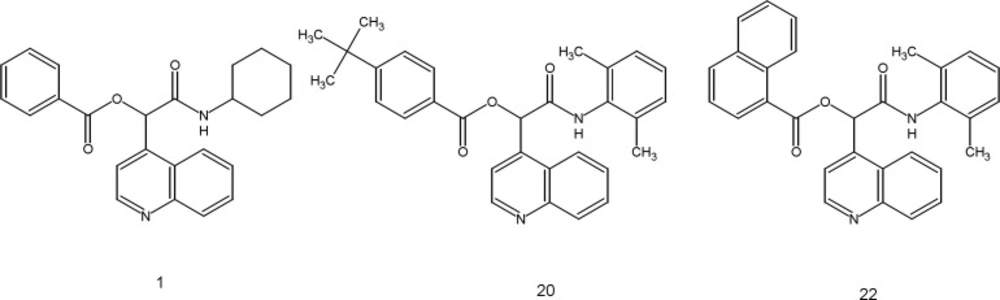
Variation of different substituents has been explored to identify the better possible combination of substituents for the improvement of antimalarial potency. To study the effect of substituents on the antimalarial activity, variation of R1 substituents have been done while keeping the R2 substituent fixed. When cyclohexyl was placed at R2, compounds having 4-tert-butylphenyl(2),4-methylphenyl(3),4-iodophenyl(8),4-cyanophenyl(9)and 1-naphthyl (10) at R1 displayed almost equal antimalarial potency. Placement of 4-chlorophenyl(4),3-chlorophenyl(5),4-fluorophenyl(6)and 4-bromophenyl (7) significantly decreased the inhibitory activity as compared to the 2 and 3. When tert-butyl was placed at R2, mentioned substituents at R1,showed IC50 from 3 to 6.9 μM in compounds 11-18. Putting the 2, 6-dimethylphenyl at position R2, with phenyl (19) and 4-chlorophenyl (21) substituents at position R1 giving IC50 4.314 and 4.177 μM, respectively. Among the 22 evaluated compounds of the series, compounds 1 (IC50 =1.511), 20 (IC50 =1.373) and 22 (IC50 =1.325) showed the better antimalarial potency. In this compounds R1 and R2 are: 1(phenyl, cyclohexyl), 20 (4-tert-butylphenyl, 2,6-dimethylphenyl),and22(1-naphthyl, 2,6-dimethylphenyl), respectively (Figure 2). These findings showed that compounds 1, 20 and 22 can be considered as new drug candidates for further evaluations in the next step of malaria drug discovery approaches.
Conclusion
In conclusion, throughout the present study, we report the preliminary results regarding the structural requirements for the antiplasmodial activityofα-(acyloxy)-α-(quinolin-4-yl) acetamides.
The present results bring essential elements, which will be used for the synthesis of more active derivatives of these compounds.