Introduction
Envenomation by arachnids causes significant injuries all over the world (1). Scorpion sting is the most important type of arachnid envenomation resulting in adult morbidity and pediatric mortality (2). Lethal scorpions mostly belong to the Buthidae family (3), among which, species of the genera Androctonus, Leiurus, and Mesobuthus are mainly responsible for envenomation in Turkey (4).
Although Mesobuthus eupeus has been considered to be a single species for many years, two subspecies of M. eupeus, which have been identified as M. eupeus phillipsii and M. eupeus eupeus have been recorded in the Turkish scorpiofauna recently by Kovařík et al. (5). Subsequently, Mirshamsi et al. (6) revised subspecies of M. eupeus existing in Iran that include the subspecies M. eupeus phillipsii and M. eupeus eupeus, and M. eupeus phillipsii elevated to species level as M. phillipsii.
Mesobuthus gibbosus occurs throughout Anatolia, except for the Black Sea coast in northern Turkey, European Turkey, Eastern and South-eatern Anatolia, and is the most abundant scorpion in this region (7-10). Mesobuthus is one of the most widely distributed genera of the family Buthidae, with species being present throughout Turkey. Stings by Mesobuthus species are more frequent than those by other scorpion species (4, 11-13). The previous reports also indicated that those Mesobuthus species are involved in numerous serious and deadly envenomations, especially in children (14–18).
Scorpion anti-venom is the only specific treatment for scorpion envenomation and is still widely used in many countries (19, 20) as there are no vaccines or other effective agents against animal venoms (21). However, anti-venoms are still administered empirically and consequently their efficacy is controversial, particularly in the case of mild or moderate scorpion envenomations (22). As venom is a complex mixture of antigens wherein not all components are equally important for the production of neutralizing antibodies, the identification of immunogenic protein(s) and/or their neutralizing epitopes may lead to the use of more clearly defined substances as immunogens to develop efficient anti-venoms. RSHA anti-Ac has been produced at the Refik Saydam Public Health Agency (RSHA), Ankara in Turkey since 1942 by venom of A. crassicauda and administering to horses (23, 24). Consequently, identification of the factors that lead to effective antivenom generation is important. With the median lethal doses of M.gibbosus (12), M.eupeus (25) scorpion venom and the potency of RSHA antivenom previously determined, there was no confirmed cross-reactivity of the three venoms with the horse anti-venom produced by RSHA, Turkey. We studied venom samples obtained from two different Mesobuthus which were Mesobuthus gibbosus from the West of Turkey and M.eupeus eupeus from Southeastern Turkey.
The present study is aimed to determine the paraspecific effects and potency of RSHA anti-Ac against the venom and determine the protein profiles (molecular weight), minimal lethal dose and also to show in-vivo effects in mice to improve quality and paraspecific activity of national antivenom.
Experimental
Scorpions
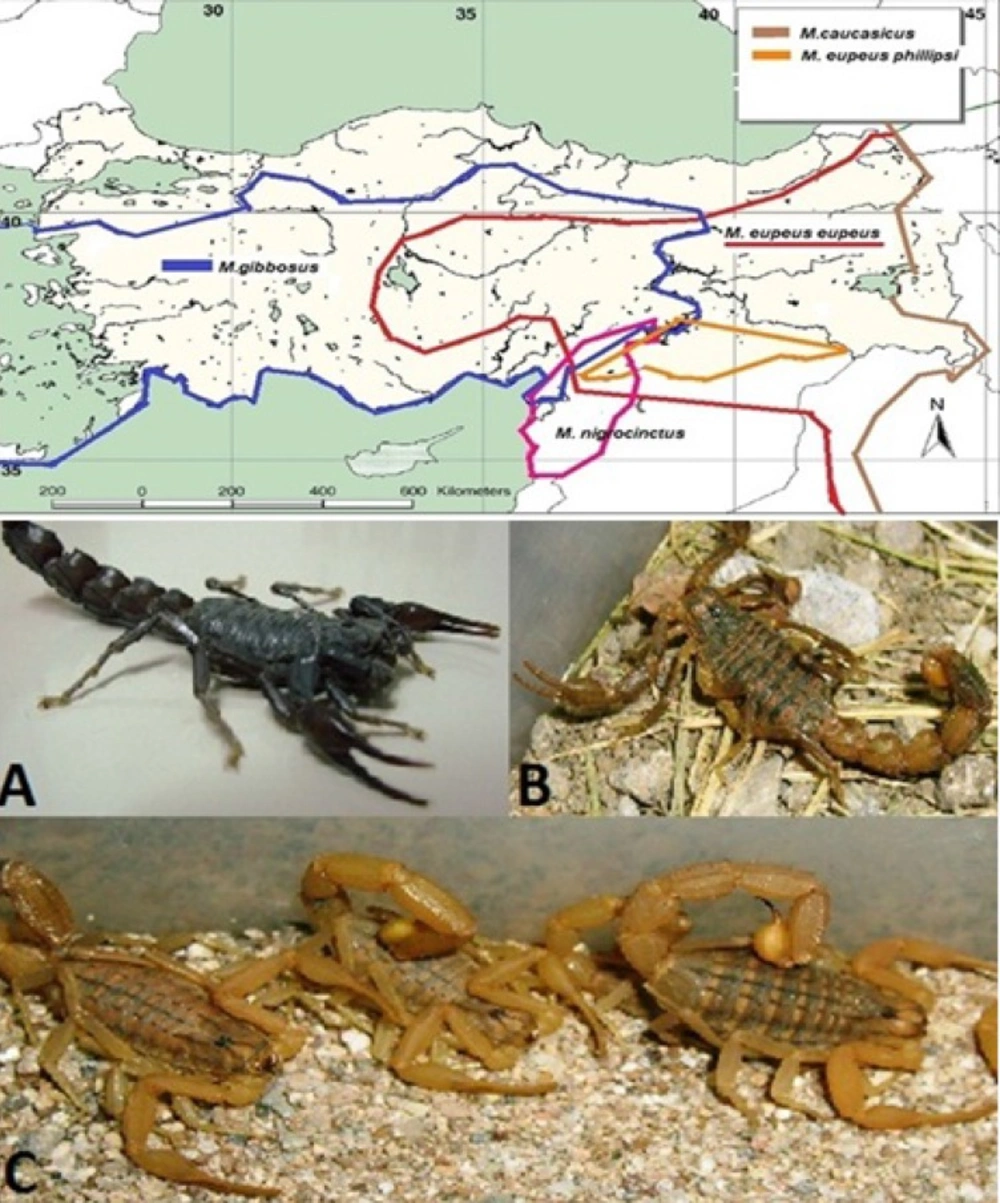
M.gibbosus was collected from Aydın, M.e.eupeus from Karacadağ Mountain (Şanlıurfa, 37°44› 45»N 39°49›50»E, 1752 m) and A.crassicauda from Şanlıurfa province at night, by using a UV lamp (Figure 1). Avoiding cannibalism, captive scorpions were housed in individual boxes at the Department of Entomology, Faculty of Veterinary Medicine, Ankara University, Turkey. The scorpions were fed with crickets or cockroaches and received water daily.
Venoms
Venom was obtained from mature M. eupeus eupeus, A. crassicauda scorpions (Figure 1) from Şanlıurfa province and M. gibbosus scorpions from Aydın province by electrical stimulation of the telson (26). The venoms were dissolved in sterile double-distilled water and centrifuged at 14,000 rpm for 15 min at 4 ºC. The supernatant was stored at -20oC. The protein content of the venoms was determined by the Bradford method using BSA as the standard (27).
Antivenom
Horse RSHA anti-Ac, produced by the Refik Saydam Public Health Agency is the unique antivenom available in the country and has been used to treat all scorpion stings in the country since 1942 (4). Dose is normalized to neutralize two minimal lethal doses (MLD) in rats when they are tested subcutaneously (4).
Experimental animals
Experimental protocols followed the guidelines of the Bioethics Commission, the RSHA, and were approved by this Commission. Swiss mice of both sexes were employed to determine the median lethal dose (LD50) and the median effective dose (ED50) by intravenous (IV) and subcutaneous (SC) route of administration. They were breed in animal facility of the RSPHA. The animals were housed under controlled temperature (20±2°C), with a 12:12 light/dark schedule and were fed with commercial rodent pellets and water ad libitum throughout the experiment.
Map of distribution of Mesobuthus speciesin Turkey (Blue line; Mesobuthus gibbosus, red line; Mesobuthus eupeus eupeus, orange line: M. e. phillipsii, brown line: M.caucasicus, green line: M.nigrocinctus ). The venom of A. crassicauda (A) has been used in the production of antivenom. M. e. phillipsii was commonly found in the Southeast Anatolia Region, but M.eupeus eupeus (B) was reported only in the Karacadag Mountain (Red circle) within the region of Turkey (Kovařík, et al., 2011). Mesobuthus gibbosus (C) is the source of many scorpion envenomation cases in the Aegean, Mediterranean, Marmara and Central Anatolia regions of Turkey
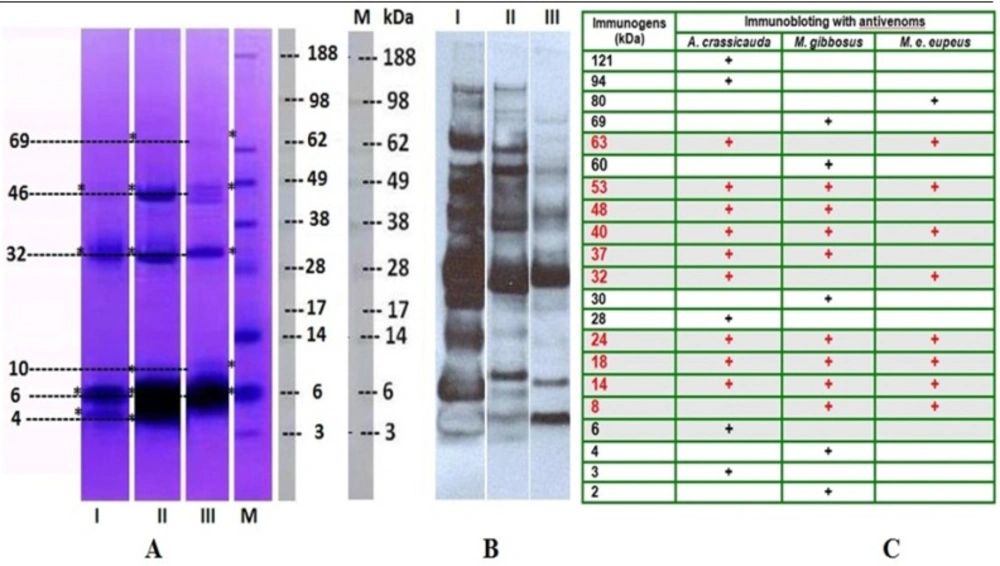
(A) The proteins of the venom of M. e. eupeus (Lane I), M. gibbosus (Lane II) and A. crassicauda (Lane III) were separated by using 4%-12% NuPAGE gradient gel electrophoresis. (B) Immunoblotting was carried out to evaluate the reactivity of the scorpion venoms components against anti-A. crassicauda horse antivenom (1: 4000), and the immunogenic compounds in both venom samples were determined. A. crassicauda venom was used as control. Lane M: Molecular weight markers –188 KDa Myosin, 98 KDa Phosphorylase, 62 KDa BSA, 49 KDa Glutamic Dehydrogenase, 38 KDa Alcohol Dehydrogenase, 28 KDa Carbonic Anhydrase, 17 KDa Myoglobin Red, 14 KDa Lysozyme, 6 KDa Aprotinin, 3 KDa Insulin, B Chain (SeeBlue® Plus2 Pre-Stain). (C) After immunoblotting, molecular weights of immunogenic proteins in all venom samples were calculated with Molecular Imaging Software
Lethal potency of the venoms
The lethality of the venom was determined as described by Behrens and Karber (28) using Swiss mice. For per each dose group, five mice were injected with increasing volume doses of M. eupeus eupeus venoms (IV injection: 28.6, 30.1, 33.2, 35.4 µg / 16g mouse [n: 20 mice]), M. gibbosus venoms (SC injection: 7.6, 11.3, 14.9, 18.5 and 22.1 µg / 20g mouse [n: 25 mice]) and A. crassicauda venom (SC injection; 3.15, 3.49, 3.89, and 4.14µg/ 20g mouse [n: 20 mice]) diluted in physiological saline solution (PSS: 0.85% NaCl). An equivalent volume of PSS was injected into five mice as negative control group. Deaths occurring after 24 h were recorded in order to determine the median lethal dose. The lethalty was expressed as the LD50.
Antivenom potency assay
Neutralization studies were carried out by mixing a constant fixed amount of RSHA anti-Ac (1 mL) with various dilutions of M. eupeus eupeus venoms (IV injection: 10, 14, 18, 25 and 28LD50 [n: 30 mice]), M. gibbosus venoms (SC injection; 29, 31, 33, 35, 38 and 40LD50 [n: 36 mice]) and A. crassicauda venoms (SC injection; 10, 20, 30, 40 and 50LD50 [n: 30 mice]) and the mixture was incubated for 30 min at 37 °C. In the control groups, mice (each group n: 2) were injected with 2 LD50, each of venom dissolved in PSS without RSHA anti-Ac. The numbers of surviving mice were recorded up to 48h. Neutralization capacity of RSHA anti-Ac was expressed as ED50, which corresponds to 1 mL RSHA anti-Ac in which the activity of the venom was reduced by 50%.
Assay of the envenomation
The mice injected with the scorpion venoms were used for lethal potency determination and they are observed for the assessment of their symptoms from the moment of the injection until the end of experiment.
Gel electrophoresis of the venoms
Polyacrylamide gel electrophoresis of venom sample was carried out by following LaemmLi (29) method. For separation of proteins, samples were run on NuPAGE® Novex® 4-12% gradient Bis-Tris gel (Invitrogen) in MES SDS Running Buffer (Invitrogen - 50mM MES, 50mM Tris-HCl, 1% SDS, 1.025mM EDTA) using Xcell SureLock Mini Cell (Invitrogen) by following standard manufacturer protocol. SeeBlue® Plus2 Pre-Stained Standard (Invitrogen) was run in parallel in order to calculate the molecular weights of proteins. Detection of proteins was carried out initially by Coomassie blue staining. The gel was then scanned and molecular weights of the proteins were calculated with Molecular Imaging Software (Kodak MI).
Immunoblotting
Western blotting was performed to determine immunogenic compounds in the venom samples. Scorpion venoms of Androctonus crassicauda (15µg), M.gibbosus (15µg), and M. eupeus eupeus (20µg), were applied to NuPAGE Novex 4–12% Bis–Tris gradient gel (Invitrogen). Proteins were transferred onto iBlot gel transfer stacks polyvinylidene fluoride (PVDF) membrane (Invitrogen) using the Blot dry blotting system (Invitrogen). Blots were then blocked with 5% skimmed milk powder in Tris-buffered saline (TBS, pH:7.5) containing 0.1% Tween20 (TBS-T) for 1 h at room temperature (RT). After the membrane was washed three times with TBS-T, it was incubated at RT with 1: 4000 dilution of the antivenom. Blots were washed and then incubated with horseradish peroxidase-conjugated anti-horse antibody and HRP- conjugated anti-rabbit (1: 3000) for 60 min at RT. PVDF membrane was again washed with TBS-T and antigens were visualized by using the Immun-Star HPR Chemiluminescent subtrate (BioRad). The membranes were exposed to X-ray film and developed in a darkroom.
Results
The identity of the scorpions were confirmed as A. crassicauda, M. gibbosus, and M. eupeus eupeus (Figure 1) using a stereomicroscope.
Lethal potency of the scorpion venom
Protein content of M. e. eupeus, M.gibbosus and A. crassicauda scorpion venom was found to be 3.72, 1.15 and 1.54 µg protein/ µl, respectively. The median lethal dose for M. eupeus eupeus, M.gibbosus and A. crassicauda scorpion venoms was determined in mice. The LD50 of M. eupeus eupeus was determined as 1.92 mg/kg by i.v injection route. The LD50 of M.gibbosus and A. crassicauda scorpion venoms were found as 0.67 mg/kg and 0.24 mg/kg by s.c injection route, respectively.
Assessment of the experimental envenomation after venom injection
Similar symptoms were observed in mice during LD50 determination. After all venom injections, mice showed some symptoms such as mastication, mouth rubbing, squeaking and fight, restlessness, aggressive behavior, humpback, tremor, tachypnea, deep dyspnea, excessively hypersalivation and lacrymation, weakness, convulsions, paralysis, coma resulting in death.
The neutralizing capacity of Androctonus crassicauda antivenom
To assess the efficacy of the monovalent antivenom, increasing doses of the venom were used while the amount of the antivenom (1 mL) was kept constant. Neutralization capacity of one mL antivenom was found to be against 23 LD50 of M. e. eupeus, 32 LD50 of M.gibbosus and 42 LD50 of A. crassicauda venoms while all the control mice died.
Determination of protein profiles
The protein profiles of the scorpion venoms were analyzed by NuPAGE® 4-12 % Bis-Tris gel, and followed by Coomassie blue staining. Proteins of the venoms were determined between 3 and 188 kDa by electrophoresis on gradient gel as shown in Figure 2-A. In the electrophoretic analysis, M. eupeus eupeus scorpion venom showed protein bands as ~ 4, 6, 32, and 46kDa (Figure 2-A; Lane I), and detected protein patterns of M.gibbosus scorpion venom were as ~ 4, 6, 10, 32, 46 and 69kDa (Figure 2-A; Lane II), and those of A.crassicauda scorpion venom as ~ 6, 10, 32, 46 and 69kDa (Figure 2-A; Lane III), after staining with Coomassie Blue. When they are compared, they showed only four similar protein bands ~ 4kDa, 6kDa, 32kDa and 46kDa (Figure 2-A) between the Mesobuthus species venoms. The common protein bands were also ~ 6, 32, 46kDa among three venom samples.
RSHA anti-Ac strongly showed reaction with both the specific venom and the other two Mesobuthus species venoms. As shown in Figure 2B, specific antibody showed reaction with the M. e. eupeus venom (3, 6, 14, 18, 24, 28, 32, 37, 40, 48, 53, 63, 94 and 121 kDa proteins), (Figure 2-B, Lane I), and with the M. gibbosus venom (4, 8, 14, 18, 24, 32, 40, 53, 63 and 80 kDa proteins), (Figure 2-B, Lane 2) and also with the A.crassicauda venom (2, 4, 8, 14, 18, 24, 30, 37, 40, 48, 53, 60 and 69 kDa proteins), (Figure 2-B, Lane 3). Immunoblotting indicated 20 proteins as immunogens in three venom samples. The common immunogen proteins were 8, 14, 18, 24, 40, and 53kDa between M. e. eupeus venom and M. gibbosus venom according to the result of immunoblotting. Comparison of western blotting profiles of all venom samples showed proteins with similar molecular weights 14, 18, 24, 40 and 53kDa (Figure 2-C).
Discussion
M. eupeus is found in Armenia, Azerbaijan, Georgia, Iran, Russia, Turkey and Turkmenistan. M. gibbosus is known to be present in Albania, Bulgaria, Macedonia, Montenegro, Greece and Turkey (5, 10, 30, 31). Toxicity of the venom and the signs of the scorpion envenomation depend on major factors such as scorpion species, venom composition and the victim´s physiological reaction to the venom (4, 32).In the study carried out in 2005, a total of 24, 261 cases of scorpion sting were reported (13). The members of genus Mesobuthus is mostly responsible in these stings in the country (33). Analysis of data in the literature showed fatal cases, especially in children, reported from different provinces of Turkey, where distribition of both the Mesobuthus species ranges (14–18). In Asian countries, most of the victims had been affected by M. eupeus (34–37). Therefore, M.eupeus is one of the major species responsible for scorpion sting in Asian countries. Lebez et al. (38) found that toxicity of M. gibbosus was 0.4 mg for mice and 2.4 mg for rats.
On the other hand, Özkan (39) obtained venom from macerated M.gibbosus telson to determine lethality reported 10 mg/kg lethal dose by s.c injection route. In current study, the median lethal dose of M.gibbosus scorpion venom was found to be 0.67 mg/kg by s.c. injection route. Our current study confirmed that A. crassicauda venom is three times more lethal than M.gibbosus venom as previously reported by Özkan et al. (12). Therefore, M.gibbosus is known as a potentially dangerous European scorpion (38). This study showed that M.eupeus and M.gibbosus could be medically important scorpions for humans and particularly children.
Zayerzadeh et al. (40) reported that lethal dose of M.eupeus venom from Iran notified 4.5mg/kg and the venom injection in rabbits evoked severe pulmonary edema and death. Latifi and Tabatabai (41) found that LD50 of M. eupeus scorpion venom from Iran was 1.36 mg/kg while Hassan (42) found it to be 1.45 mg/kg by i.v injection route. Özkan and Carhan (25) determined that the median lethal dose for M. eupeus from the Central Anatolian region of Turkey to be 0.18 mg/kg by i.c.v. injection route. They stated that M. eupeus scorpion venom is 11 times less toxic than A. crassicauda scorpion venom. In our study, LD50 of M. e. eupeus was determined that to be 1.92 mg/kg by i.v. injection route. We found similar results among toxcitiy of A. crassicauda venom and M.e. eupeus venom. Mice, experimentally envenomed with M. gibbosus venom, manifested tremor, hypersalivation, mouth and nose bleeding, paralysis and death (39). In our study, similar sympathetic and parasympathetic signs except for mouth and nose bleeding were observed in mice during lethality determination,
All scorpion species with high potential of mammal specific neurotoxins belong to the Buthidae family, due to their neurotoxic effects and medical importance (2). Scorpion venoms can be classified into two groups according to their molecular weights, long-chain (6,500 to 7,800 Da) and short-chain neurotoxins (3,000 to 4,400 Da) (43-45). Uçar and Taş (46) indicated that protein bands’ molecular weight ranges from 6.5 to 210 kDa detected in the venom of M. gibbosus from Manisa province. In current work, analysis of electrophoresis shows that all scorpion venoms possess low molecular weigth proteins. There has been antigenic similarity among the venom of scorpions belonging to the Buthidae family (47). Ozkan et al. (48) reported that three protein bands with molecular masses of 70, 87 and 100 kDa were detected in M. gibbosus and M. eupeus scorpion venom samples, according to the SDS-PAGE analyses. Ozkan and Ciftci (33) indicated that the protein bands with molecular masses of 28, 30, 33, 68 and 98 kDa were detected in the venom of M. gibbosus from Mugla province. In the current study, western blotting showed that RSHA anti-Ac strongly reacted with A.crassicauda, M. gibbosus and M. e. eupeus scorpion venoms which have antigenic similarity (Five protein bands with molecular masses of 14, 18, 24, 40, 53 kDa).
Altınkaynak et al. (14) recommended that the Turkish antivenom (A.crassicauda antivenom) is used to treat M. gibbosus envenomation cases, since it had been effective in 91.6% of the cases which were studied. The antivenom was not effective in only two cases (9 and 13-month-old infants) because the patients had late received the first aid and hospitalization, and the sting site was on the neck (14). Soker and Haspolat (49) verified that the Turkish antivenom was effective in 90.3% of all scorpion related cases in South-Eastern Anatolia. In many studies, it was reported that A. crassicauda antivenom was capable of neutralizing various scorpion venoms in mice (12, 23, 50, 51). In our study, bioassays showed similar paraspecific activity with previous potency studies. We demonstrated that 1 mL of the antivenom can neutralize 23 LD50M. e. eupeus, 32 LD50M. gibbosus and 42 LD50A. crassicauda venom in mice.
Conclusion
Lethal potency of the venoms indicated that M.e.eupeus and M.gibbosus could be dangerous for people, especially children. These scorpion venoms possess low molecular weight of proteins according to the analysis of electrophoresis. The western blot analysis also shows that three venoms have similar antigenicity pattern. RSHC anti-Ac (1 mL) was capable of neutralizing M. e. eupeus venom (23LD50), M.gibbosus venom (32LD50) and A. crassicauda venom (42LD50). The RSHC anti-Ac can be used in the treatment of envenomation by M. e.eupeus and M.gibbosus scorpion stings.