Introduction
Dorzolamide hydrochloride (DRZ) was synthesized in 1980 and it was the first carbonic anhydrase inhibitor approved for the topical treatment of glaucoma in humans in 1995. The drug reduces the production of bicarbonate ions, thereby reduces secretions and lowers intraocular pressure (1, 2). Elevated IOP may lead to glaucoma in patients with ocular hypertension. Nowadays due to their higher efficiency and lower systemic adverse reactions, ophthalmic preparations are considered to be the first choice in glaucoma treatment (3, 4).
One of the most important problems of the ophthalmic drops is their short ocular residence time. The drug is quickly diluted by tears and is removed through nasolachrymal ducts (5). Therefore, it is beneficial to design a biocompatible ocular drug delivery system that can increase the residence time of the drug in the eye.
Liposomes are self-assembled colloidal particles; consist of lipid bilayers surrounding an aqueous compartment. Due to their cell-like nature, liposomes are able to attach to cellular tissues of the body. Today, nanotechnology provides smaller dimension of liposomes; under the name of nanoliposomes (6, 7). The small dimensions provide greater surface activity and adsorption on the surface of cornea. Drug-loaded nanoliposomes can maintain a relatively constant drug concentration, increase the drug residence time in the eye and consequently enhance therapeutic efficiency; therefore, lower doses of the drug with less frequency can be applied (8, 9).
The objective of this study is to develop ocular DRZ – nanoliposomes and evaluate their potential use for the treatment of ocular hypertension.
Experimental
Materials
Dorzolamide HCl supplied by BASELUX (S.A-Switzerland), Soy phosphatidylcholine (phospholipon 85G®) purchased from Lipoid (Germany) and cholesterol supplied by Sigma (Germany) were used for the preparation of formulations. All other materials were of the highest grade commercially available.
Preparation of DRZ – nanoliposomes
Reverse-phase evaporation vesicle (REV) method: The lipid components, with 7:3 and 7:4 molar ratio of soy phosphatidylcholine (SPC): cholesterol (Ch), were dissolved in chloroform-methanol solution (2:1 v/v). The organic solvent was evaporated at 65 oC under reduced pressure at 150 rpm using a rotary evaporator (EV311, Lab-Tech, Germany) and the thin lipid film obtained was maintained at 4 oC for 24 hours to ensure complete removal of solvents. The film was dissolved in diethyl ether, followed by addition of 10 mL phosphate buffer (pH 5.8) containing 2% (w/v) DRZ. The organic solvent was removed at 37 oC and the dispersion was sonicated for 30 min using a bath sonicator (T710, Elma, Germany). To ensure full lipid hydration and maturation, the nanoliposomal dispersion was kept at 4 oC overnight (10).
Thin layer hydration (TLH) method: In this method the thin lipid film obtained by previously described conditions was directly hydrated with phosphate buffer (pH 5.8) containing DRZ (2% w/v), sonicated and kept at 4 oC overnight for maturation (11).
All prepared formulations were sterilized by filtration (0.22 μ).
Characterization of DRZ – nanoliposomes
In order to determine the encapsulation efficiency (EE%) of DRZ – nanoliposomes, the formulation was transferred into centrifugal filter device (Centritrep®-50 KDa), and centrifuged at 3000 rpm and 25 °C±1 for 30 min (Clements 2000, Germany). Then, the supernatant was analyzed for free DRZ using UV spectrophotometer (Biochorm, England) at 254 nm. EE% was calculated as follows:
The following parameters were measured: The mean particle size, polydispersity index (PDI) and zeta potential of the nanoliposomes by nanosizer (Nano ZS, Malvern, UK), viscosity by Brookfield viscometer (Model DV-II+Pro, Middleboro, USA) at 100 rpm, surface tension by the De Nouy ring method (CSC Scientific Company, USA), pH value, and refractive index (RI) at 25±1 °C.
In-Vitro Drug Release Study
Nanoliposomal samples enclosed in dialysis bags (cellulose membrane MW cut- off 12 KDa, Sigma) were immersed in 250 mL phosphate buffer (pH 7.4) at 25±1 °C and were stirred at 100 rpm. At predetermined time intervals, samples were withdrawn and analyzed for DRZ spectrophotometrically at 254 nm. DRZ solution (2% w/v) was also tested as control experiment (12).
Ex-Vivo Drug Permeation Study
The experiment was carried out using Franz diffusion cells designed for ocular permeation studies with receiver medium of 10.5 mL phosphate buffer (pH 7.4) at 35±1 °C. Albino rabbits were sacrificed by IV injection of sodium phenobarbital and the whole eyes were enucleated. The cornea was excised and then mounted on the diffusion cell. Nanoliposomes were applied in the donor side. Samples (1 mL) were withdrawn at specific time intervals and assayed spectrophotometrically at 254 nm. The receiver medium was immediately replaced by fresh buffer. The permeation behavior of free drug as a control experiment was determined using DRZ solution (2% w/v).
The steady-state flux (Jss) of different formulations was determined by the slope of the linear portion of the plots of the amount of drug in the receiving chamber versus time, divided by exposed corneal surface area and the lag time that was estimated from the x-intercept of the linear portion of the graph. Apparent corneal permeability coefficient (Papp) was calculated according to the following equation:
Cd represents the drug concentration in the donor compartment (13).
In-Vivo IOP Measurement
The animal experiments were conducted in full compliance with regulatory principles of ethics committee of Ahvaz Jundishapur University of Medical Sciences. Albino rabbits (2.5–3 Kg) were housed at controlled temperature (25±2 °C), and humidity (60±5%), with a 12/12-h light-dark cycle. The animals were divided into three groups of six rabbits. Group I received the selected DRZ – nanoliposome, group II was treated with DRZ solution and group III received Biosopt® ophthalmic drop (Bakhtar Biochemie Co, Iran). One drop (0.05 mL) each of DRZ – nanoliposome, DRZ solution or Biosopt® drop was instilled into the right eye, while the non-treated eye was considered as control. IOP of both eyes was determined using IOPEN® tonometer for 8 h. The mean of three consecutive tonometric measurements was calculated for each animal (7).
Stability Study
REV and TLH nanoliposomes with lipid molar ratio of 7:4 were stored at 4 ºC; their particle size and EE% were monitored after 1 and 3 months (14).
Statistical Analysis
All studies were carried out in triplicate and data were reported as a mean ± SD. One-way ANOVA and multiple comparison Tukey’s test were used to assess the significance of the differences between the various groups, p<0.05 was considered statistically significant.
Results
Characterization of DRZ – nanoliposomes
The data showed that nanoliposomal formulations with lipid ratio of 7:4 (SPC:Ch) produced significantly higher EE% than those with lipid ratio of 7:3 (SPC: Ch) (p<0.05) (Table 1). Particle size results showed that higher proportion of cholesterol in lipid ratio led to form larger particles (p<0.05) but they were still below 100 nm and in acceptable range (Table 1). Comparing the results of formulations with same molar ratio, revealed that nanoliposomes prepared by TLH method had significantly smaller particle size than those made by REV method (p<0.05). PDI is a measure of dispersion homogeneity and ranges from 0 to 1. Values close to zero indicate a homogeneous dispersion while those greater than 0.3 indicate high heterogeneity (15). PDI of all formulations in this study were approximately 0.3 which indicates acceptable uniformity and homogeneity of the formulations (p>0.05). Nanoliposomal formulations 7:4 (SPC:Ch) REV and 7:4 (SPC:Ch) TLH were positively charged with zeta potential value of +12.8 and +10.3 mV, respectively. The tonicity of the formulations was equivalent to that of a 0.875% sodium chloride solution.
| Liposomal Formulation | Particle size (nm) | Polydispersity index | Encapsulation |
|---|---|---|---|
| SPC:Ch (7:4) / REV | 76.14±19.24 | 0.340±0.015 | 57.51±4.02 |
| SPC:Ch (7:4) / TLH | 19.93±5.23 | 0.340±0.016 | 67.36±2.27 |
| SPC:Ch (7:3) / REV | 19.72±6.43 | 0.340±0.015 | 6.14±1.88 |
| SPC:Ch (7:3) / TLH | 5.68±2.42 | 0.330±0.016 | 2.11±1.20 |
The results of viscosity, surface tension, pH and RI values are presented in Table 2. The viscosity values were between 40 to 46 cps. The results showed that method of preparation and lipid molar ratio did not influence the viscosity value of nanoliposomes (p>0.05). The surface tension of the formulations in decreasing order is: Biosopt® > REV prepared DRZ – nanoliposomes > TLH prepared DRZ – nanoliposomes (p<0.05). The pH values of DRZ – nanoliposomes were between 5.61- 5.63. There were no significant differences among them (p>0.05). The pH value measured for Biosopt® was 5.2 which is significantly lower than that of DRZ – nanoliposomes (p<0.05). The RI values of DRZ –nanoliposomes were 1.343 which is close to that of tears.
| Liposomal Formulation | Viscosity (cps) | Surface tension (N/m) | pH | Refractive index |
|---|---|---|---|---|
| SPC:Ch (7:4) / REV | 42±0 | 0.054±0.003 | 5.62±0.02 | 1.3433±0.0001 |
| SPC:Ch (7:4) / TLH | 46±3 | 0.047±0.005 | 5.63±0.01 | 1.3433±0.0001 |
| SPC:Ch (7:3) / REV | 44±6 | 0.055±0.004 | 5.61±0.01 | 1.3433±0.0002 |
| SPC:Ch (7:3) / TLH | 40±3 | 0.047±0.004 | 5.63±0.01 | 1.3435±0.0002 |
| Biosopt® | 96±3 | 0.070±0.005 | 5.20±0.01 | 1.3418±0.0001 |
In-Vitro Drug Release
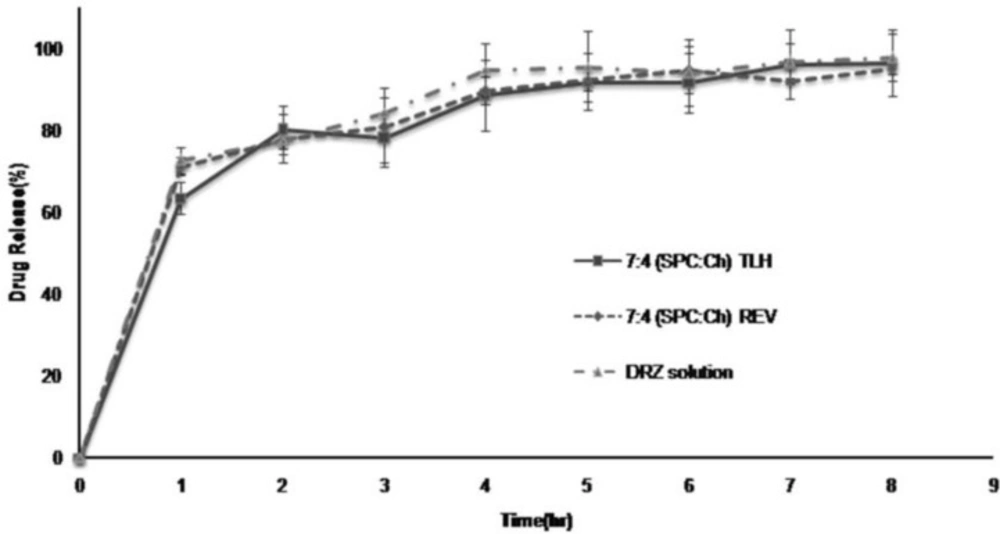
This study was carried out only with 7:4 (SPC:Ch) REV and 7:4 (SPC:Ch) TLH DRZ –nanoliposomes which had good in-vitro properties especially regarding EE%. All of the experiments were performed without separation of entrapped and non-entrapped DRZ. The release profiles of DRZ from DRZ – nanoliposomes and DRZ solution shown in Figure 1 are almost the same. A burst effect of drug release observed during the first hour (about 70%) that followed by nearly complete release (over 95%) after 6 hours.
Ex-vivo Drug permeability
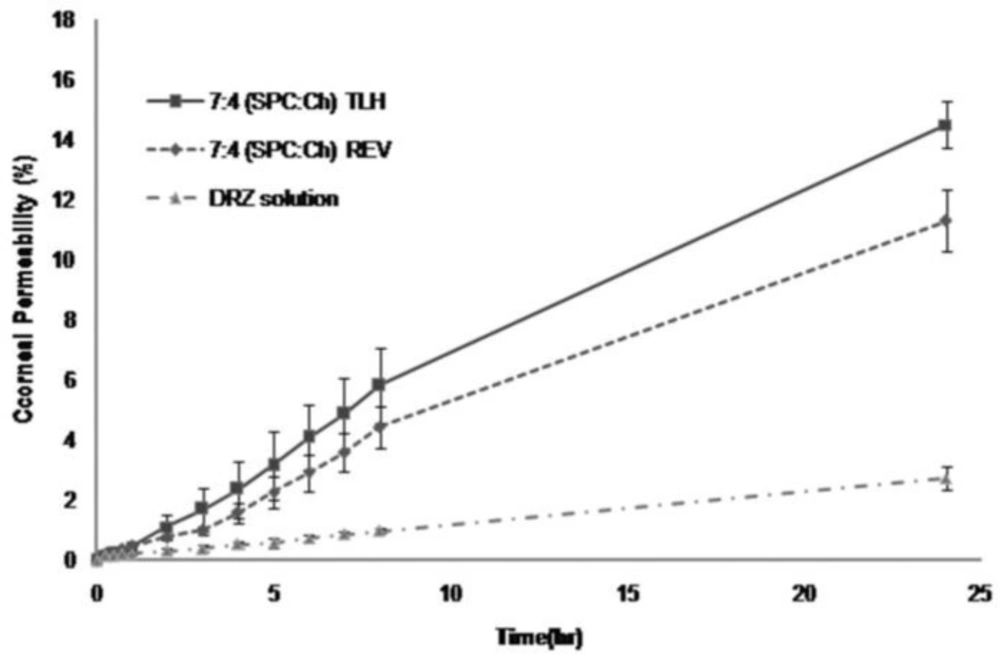
After 8 and 24 h, corneal permeability of DRZ for 7:4 (SPC:Ch) REV was 4.5 and 11%, for 7:4 (SPC:Ch) TLH was 6 and 14.5%, and for DRZ solution was 1 and 3%, respectively (Figure 2). Values of DRZ – nanoliposomes were significantly higher than those of DRZ solution (p<0.05). Significant (p<0.05) differences were observed between permeability results of 7:4 (SPC:Ch) REV and 7:4 (SPC:Ch) TLH in favor of TLH prepared DRZ – nanoliposomes.
Papp, Jss and lag time values are shown in Table 3. DRZ – nanoliposomes showed permeability value about 5-7 fold higher than DRZ solution. However, the permeability parameters (Papp and Jss) of 7:4 (SPC:Ch) REV and 7:4 (SPC:Ch) TLH were not significantly different (p>0.05). A lag time of about 40 min was observed for DRZ – nanoliposomes, whereas DRZ solution permeated through cornea without any lag time.
| Liposomal Formulation | Papp (cm/h) | Jss (mg/cm2.h) | Tlag (min) |
|---|---|---|---|
| SPC:Ch (7:4) / REV | 2.87e-3±0.00050 | 0.0575±0.0111 | 46±12 |
| SPC:Ch (7:4) / TLH | 3.82 e-3±0.00080 | 0.0764±0.0160 | 41±18 |
| DRZ solution | 5.40 e-4±0.00001 | 0.0108±0.0003 | 0 |
Stability
The drug leakage after 1 and 3 months storage at 4 °C were found to be 1.12% and 2.86% from 7:4 (SPC:Ch) REV DRZ – nanoliposomes and 0.2% and 1.64% from 7:4 (SPC:Ch) TLH, respectively. The particle size of DRZ – nanoliposomes showed no statistically significant changes over 3 months storage at 4 °C (p>0.05).
Considering the higher permeability and the smaller particle size of 7:4 (SPC:Ch) TLH prepared DRZ – nanoliposomes as compared to REV prepared ones, the former was selected for subsequent in-vivo studies.
In-Vivo Intraocular Pressure (IOP)
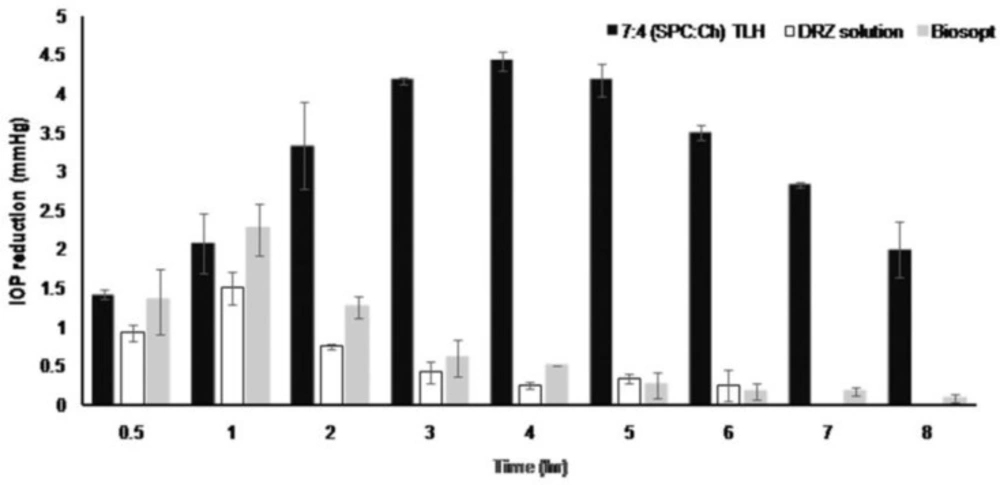
Figure 3 shows that the IOP lowering activity of marketed product and DRZ solution within 1 h after instillation reached maximum values of 2.25 mmHg and 1.5 mmHg, respectively, but this effect quickly decreased and disappeared for both products. DRZ – nanoliposomes produced a significantly prolonged effect of reduction in IOP. The maximum IOP decreased value was 4.42 mmHg at the 4th hour, which was significantly higher than those of DRZ solution and the marketed product (p<0.05). Macroscopic examination did not reveal any inflammation, redness or irritability in the rabbits’ eyes during the procedure.
Discussion
Particle size is one of the most important indicators in nanotechnology. The fine size of nanoliposomal ophthalmic preparations tends to cause less abrasion, irritation and sensitivity (6, 16). In this study, particle size of all formulations was below 100 nm. Other studies revealed that the liposomes with small size and single layer such as SUV, adhere more strongly to tissue and perform a better drug delivery (17). Calculated tonicity of the formulations was nearly close to isotonic solution which is important for formulations intended for ophthalmic use.
Marketed DRZ eye drops are in a solution form, the drug is absolutely freely soluble and becomes diluted with tears and is readily discharged from the eyes. Hence, viscosity enhancers such as cellulose derivatives are mostly used in marketed products to increase consistency and improve adherence in the eye (2). But, it should be considered that high consistency can cause problems in the movement of the eyeball, the process of optical refraction and creation of the image on the retina. The viscosity of an ophthalmic formulation should be in the range of 15–50 cps (18). In this study, formulation 7:4 (SPC:Ch) TLH prepared DRZ – nanoliposomes had a viscosity equal to 46 cps, which is in the acceptable range; while the marketed product (Biosopt®) had a significantly higher viscosity equal to 96 cps as measured in our Lab.(p <0.05).
Surface tension is another highly important characteristic. In our study, surface tension of DRZ – nanoliposomes was lower than that of Biosopt® which is attributed to the surface activity of phospholipid amphiphilic molecules (19). The lower surface tension, the better spreading of the product on the cornea and the more contact between them. But, eye drops with much lower surface tension than lachrymal fluid (0.04 to 0.05 N/m) may destabilize the tear film and cause visual problems (2). Formulation 7:4 (SPC: Ch) TLH prepared DRZ – nanoliposomes had a surface tension of 0.047 N/m.
The pH value of all formulations was around 5.6. DRZ has maximum stability in the range of pH 4-6 (20). The recommend pH range of eye drops is 3.5 - 8.5. It should be taken into consideration that a shift from neutral pH to either acidic or basic may cause eye irritation.
It is recommended that eye drops should have RI close to tears fluid (1.34 to 1.36) (2). DRZ – nanoliposomes in our study, had RI in the acceptable range (Table 2).
Higher encapsulation efficiency was obtained by increasing the content of cholesterol. As cholesterol settles between phosphatidylcholine molecules, it fills empty spaces, increases rigidity of the bilayer films and prevents leakage of the drug. Also, it stabilizes nanoliposomes against external pressure such as extreme shaking (21, 22).
In this study, the release of the drug from the DRZ – nanoliposomes did not show a significant difference compared with the drug release from DRZ solution. The high-rate of nanoliposomes drug release during the first hours may be due to the presence of available free drug in the formulations and the high concentration gradient of DRZ between the contents in the dialysis bag and the large-volume (250 mL) receiver phase.
Although DRZ – nanoliposomes and DRZ solution exhibited similar in-vitro drug release, DRZ – nanoliposome showed higher permeability through the cornea in comparison to drug solution. This can be explained in terms of the conditions under which the ex-vivo study was performed. This is more similar to in-vivo conditions in relation to the receiver medium volume (10.5 mL).
It is speculated that the high rate of drug permeability of nanoliposomes is a result of high lipophilicity of the vehicle, which leads to increase in residence time on the surface of the cornea (23). The nanoliposomes can carry hydrophilic drugs (such as DRZ) in their cores and then attach to the surface of the corneal epithelium and gradually release the drug (6, 24). Other possible mechanisms such as fusion of liposome membrane with the cornea or endocytosis of liposomes may lead to the drug release in the anterior eye space after passing through the cornea (22). In addition, the lag time observed in ex-vivo permeation studies of DRZ – nanoliposomes may support this hypothesis. DRZ – nanoliposomes surface had a positive charge because of the cationic nature of DRZ at pH below 6.4 (20). So, this would lead to their adhesion to polyanionic surface of cornea and conjunctiva and improvement in corneal retention and penetration (17, 25, 26). The greater permeability of formulation 7:4 (SPC:Ch) TLH as compared to 7:4 (SPC:Ch) REV can be attributed to their smaller particle size (23).
It was also observed that DRZ – nanoliposomes in contrast with DRZ solution and marketed product induced a remarkable decrease in IOP and with respect to the duration of action, the effect of nanoliposomes was prominent. Our findings are in accordance with the results of Hathout et al. They investigated liposomal acetazolamide and concluded that acetazolamide liposomes composed of egg phosphatidylcholine: Cholesterol: stearylamine (7:4:1) molar ratio prepared by a method almost similar to TLH method used in our study, revealed more prolonged IOP-lowering effect compared to the solution form of the drug (7).
In their in-vivo study on liposomal diltiazem as ocular drug delivery system for glaucoma, Mokhtar Ibrahim et al., proved the system enhanced IOP-reducing activity as compared to the solution form at equal concentration (27).
Hironaka et al. also investigated the uptake of carboxyfluorescein (CF) into conjuctival cells via liposomal eye drops and concluded that the amount of CF incorporated into cells increased when using liposome as a carrier; and they proposed that the increase in drug uptake was due to the affinity of phospholipid bilayer to the cell membrane that led to effective delivery of hydrophilic drug to the retina (28).
The higher permeability coefficient and flux of DRZ – nanoliposomes versus solution form provide a good explanation for this higher effectiveness and prolonged therapeutic effect (29).
Conclusions
Nanoliposomes natural structure, their positive surface charge and small vesicle size contribute to their adhesion to the corneal membrane which leads to improved clinical effect. Of the two methods that were applied to prepare nanoliposomes systems, TLH method is simpler, faster, cost effective and has less toxic solvent residue than REV method. In this study, formulation 7:4 (SPC:Ch) TLH was selected because of its small particle size, high EE% and high corneal permeability. The characteristics of this formulation in terms of surface tension, viscosity, pH, RI and safety were all in the acceptable range required for ophthalmic preparations. The formulation was stable throughout 3-months period of storage and revealed longer and higher IOP-lowering activity in comparison with DRZ solution and marketed eye drop. The prolonged effect may be explained in term of gradual drug release from nanoliposomes which are depot in the cornea, suspended in aqueous humor or attached to the surface of the anterior space. It seems that the selected formulation is a suitable candidate for human clinical trials. We recommend the addition of an antioxidant to prevent lipid oxidation and prepare a stable formulation for longer period of time. However, assay of DRZ in aqueous humor following drug instillation into rabbit eye should be done before clinical trials as well.