Introduction
Ulcerative colitis (UC) and Crohn’s disease (CD) are two major chronic inflammatory bowel diseases (IBD), that include characteristic ulcers, or open sores (1). IBD is largely attributed to the abnormal mucosal T cell response to bacterial antigens contained within the gut lumen (2). UC is considered to be a Th2-mediated disease (3). Oxazolone-induced colitis is a Th2 model with rectum pretreated by administration of oxazolone in an ethanol vehicle (4). Oxazolone-induced colitis of mice was used as the experimental model in our study because of the histological resemblance to human UC. In this model, the initial toxic effect induced by oxazolone lead to lamina propria flooding and immune response mediated by IL-4, IL-5 and IL-13.
Tetramethylpyrazine (TMP) is one of the major bioactive components extracted from Chinese medicine Ligusticum wallichil Franch. Previous studies have shown that TMP has various complex pharmacological effects including anticoagulation, expanding blood vessel, and protecting vascular endothelium. It is also demonstrated that TMP can suppress the activity of glioma cell line by inhibiting calcium influx (5), and also inhibit melanoma metastasis through suppressing VEGF activity (6). Recent studies have paid more attentions to TMP as an anti-inflammatory agent and anti-tumor agent (7, 8). Current treatments of UC are not universally effective, thus studies of therapeutic effects of TMP in UC and the underlying mechanism is necessary.
He X, et al illustrates that TMP inhibits PPAR-γ signaling in colon mucosa, and further attenuates the damage of oxazolone on mice (9). Mononuclear cells of peripheral blood, spleen and lamina propria were isolated from normal and oxzolone-treated mice for further exploring the pharmacological action of TMP on immune response. Dose-study [low dose (0.5 g/L, middle dose (1 g/L) and high dose (2 g/L)] of TMP was performed on mononuclear cells in-vitro. In the present study, we evaluated the apoptosis in mononuclear cells, and detected the expression level of nuclear factor kappa B (NF-kB), transcription factor-activated protein-1 (AP-1) and nuclear factor of activated T cells (NF-AT) which were important for T cell survival, activation and differentiation.
Experimental
Induction of colitis
This study was approved by the Ethics Review Committee of the 6th People’s Hospital affiliated to Shanghai Jiaotong University. A total of 40 healthy Kun Ming mice (7~8 week, 20~30 g) were obtained from Experimental animals breeding centre of our medical school and maintained in a specific pathogen-free (SPF) environment. Then all mice were randomly divided into 5 groups (n = 8), including normal group, low dose (0.5 g/L), middle dose (1 g/L) and high dose (2 g/L) tetramethylpyrazine-treated group and model group without the administration of tetramethylpyrazine. Except for the normal group, the mice in other groups were induced with oxazolone colitis according to Heller’s method (3). In presensitization of mice, a 2 × 2 cm field of the abdominal skin was shaved and 200 µL of 3% (w/v) solution of oxazolone in 100% ethanol was applied. Five days after presensitization, mice were rechallenged rectally with 150 µL 0.5% oxazolone in 50% ethanol and inverted to prevent blowback.
Histological assessment of colitis
Tissues, which were removed from 2 × 10 mm large intestine of oxazolone–treated mouse, were fixed in 20% neutral-buffered Formalin solution and then embedded in paraffin. Consequently, the paraffin-embedded tissues were cut into tissue sections, and stained with hematoxylin-eosin (HE). Stained sections were examined under optical microscope for the evidence of colitis. The criterion of colitis included the presence of ulcer, fibrosis, recess, hydrous, hemorrhage, pseudo membrane formation, the presence and degree of lymphocyte infiltration; eosinophilia medium-sized cell infiltration and the presence of mucoprotein reduce.
Isolation of PBMC, SMC and LPMC
Mice were euthanized 3 days after induction of colitis. Eyeballs were extracted and blood was drawn into EDTA-K2-pretreated anticoagulant tube, and then shaken up for 30s. Peripheral blood mononuclear cells (PBMC) were isolated by a density gradient centrifugation. Spleen was washed by D-hanks solution for 2-3 times, and cell suspension was filtered and transferred to a 10 ml tube. Spleen mononuclear cells (SMC) were isolated by density gradient centrifugation. Lamina propria mononuclear cells (LPMC) were isolated from freshly obtained colonic specimen using a technique described by Boirivant (10). The colonic specimens were initially washed in D-hanks solution, and incubated in the medium containing 50 mg/L amphotericin and 50 mg/L gentamicin at 37 °C for 10~20 min, and then washed in D-hanks again for 2-3 times sequentially. The tissues were cut into 1 × 1 mm pieces, and incubated two times in HBSS (0.75 mmol/L EDTA and 1 mmol/L DTT) at 37 °C for 15 min. The tissue was digested further in RPMI 1640 (400 U/ml collagenase and 0.01 DNase I) in a shaking incubator at 37 °C. LPMC was resuspended in 100% Percoll, layered under a 40% Percoll gradient, and then centrifugated to obtain the lymphocyte-enriched population at the 40-100% Percoll interface. BMC, SMC and LPMC were all seeded at 2.5 ~ 3.5 × 105 cells/ml.
Cell culture of PBMC, SMC, LPMC
Cells of PBMC, SMC, and LPMC for the evaluation of apoptotic rate were incubated for 48 h and 96 h, respectively. Then different concentrations of tetramethylpyrazine were added according to the requirements. After the 12 h treatment, cells were centrifuged at 1500 r/min for 5 min, and then washed by PBS twice. The cells were resuspended in 200 µL binding buffer at the concentration 1~2 × 105/ml and then detected by Annex in V- FITC method for evaluating cell apoptosis. Cells for the evaluation of NF-kB, AP-1 and NF-AT mRNA level were incubated for 24 h and then treated by tetramethylpyrazine according to the requirements for 48 h.
Annexin V FITC used for apoptosis detection
A volume of 200 µL cell suspension at concentration of 1~2 × 105/mL was transferred to a 5 mL culture tube, followed by the addition of 10 µL FITC Aennexin V and 5 µL PI. The cells were gently vortexed, and then incubated for 15min at room temperate in the dark. Then 300 µL binding buffer was added and the mixture was analyzed by flow cytometry within 1h.
Reverse transcription and Taqman probe labeled quantitative real-time polymerase chain reaction (RT-PCR)
RNA was extracted from 5 groups of mononuclear cells. An equivalent amount cDNA per sample was amplified by using specific Taqman probe labeled primers for NF-kB, AP-1, NF-AT and GAPDH (Table 1). PCR were performed in a total volume of 10 µL including 0.5 µL forward primer, 0.5 µL reverse primer, 0.5 µL dNTP solution, 2 µL Taq DNA polymerase, 31.5 µL DEPC- H2O and 5 µL cDNA sample. PCR reaction was carried out at 93 °C (2min), 93 °C (45sec) for 30 cycles, 55 °C (1min) for 10 cycles, 93 °C (45sec) and 55 °C (1min). ABI Prism 7000 SDS Software was used to analyze data. Copy numbers of each gene were calculated according to the standard curve. The results of mRNA levels were normalized to the housekeeping gene GAPDH.
| Gene | Primer | Product size (bp) |
|---|---|---|
| AP-1 | Forward:5’- CCCCACCCAGTTCTTGTGCC -3' | 187 |
| NF-kB | Forward:5’-TTGCCACGCACAGACGGTGT -3' | 192 |
| NF-AT | Forward:5' ACCCTGCCTTTACCCTTC 3' | 227 |
Statistical analysis
All Statistical analysis was processed by SPSS version 13.0 (SPSS Inc., Chicago, IL). Comparison between different treatments was performed by using the T test. P < 0.05 was considered to be significant for all tests.
Results and Discussion
Epicutaneous presensitization and intrarectal rechallenge with oxazolone leads to oxazolone colitis
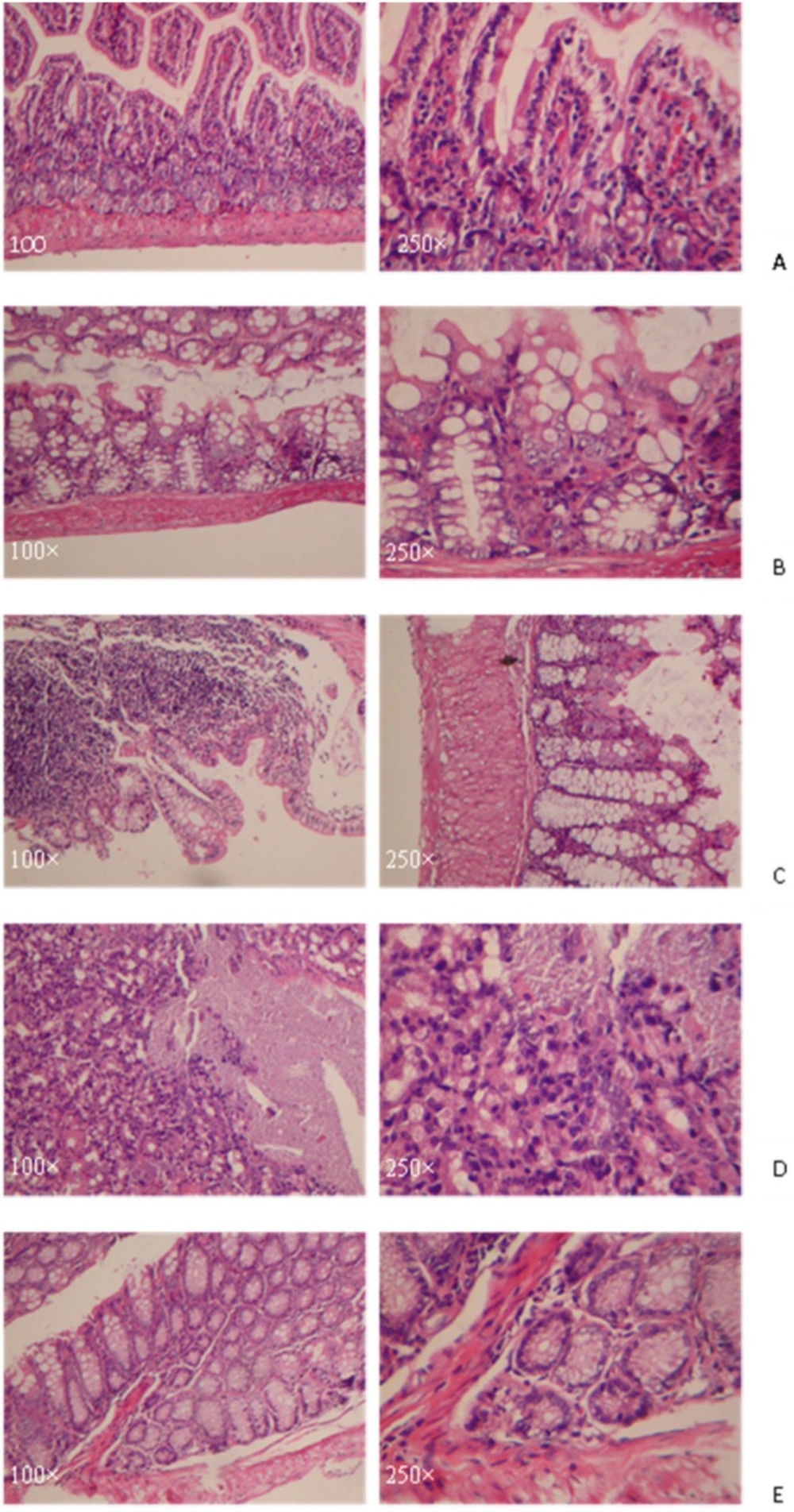
Compared with the normal group, mice in experimental groups became lazy, anorectic, and had the symptoms of visual bloody stool, and weight loss after 24 h oxazolone treatment. Histological examination of mouse colons on the third day showed that mice in model group, which developed massive bowel wall edema, developed with a loss of crypts and mucosa membrane shedding. The necrosis of intestinal mucosa membrane of those mice turned into ulcer. The dense infiltration of muscularispropria and serosal layers of the mucosa with lymphocyte were shown in Figure 1.
A: the normal group without any treatment. The glandular structure was basically normal with a small amount of bleeding and inflammatory cells infiltration.
B: Slight crypts abscess, mucosa membrane falling off to an ulcer, small fibrosis and inflammatory cells infiltration were observed.
C: Slight crypts abscess, mucosa membrane falling off to an ulcer were observed. The ulcer was mainly located in the mucous layer with a lot of inflammatory cells infiltration.
D: The glandular structure disordered. Mucosa membrane became necrosis and falling off and erosions.
E: The glandular structure was normal. Crypts abscess and inflammation cells infiltration were observed.
Inhibition of oxazolone-induced colitis by tetramethylpyrazine is associated with increased apoptosis of mononuclear cells in spleen and lamina propria
The apoptotic rate of SMC, LPMC were significantly lower (P < 0.05) in oxazolone-induced colitis model group than that in normal group (Table 2). There was no significant difference in PBMC apoptosis between normal, model group and the tetramethylpyrazine-treated groups (P > 0.05). Compared with the model group, treatment with tetramethylpyrazine resulted in an increase of SMC and LPMC apoptosis. In middle dose group, apoptotic rate of LPMC which was cultured for 48 h and 96 h were both significantly higher than that in the model group (P < 0.05), but still lower than that in normal group. There were higher SMC apoptotic rates in high dose group and normal group than that in the model group after both 48 h and 96 h culture.
| Group | 48 h | 96 h | ||||
|---|---|---|---|---|---|---|
| SMC | LPMC | PBMC | SMC | LPMC | PBMC | |
| Normal | 4.61±1.19 | 4.23±1.01 | 3.97±0.89 | 3.63±0.46 | 4.93±0.71 | 3.60±0.46 |
| Model | 2.89±0.62* | 0.58±0.21** | 2.69±0.92 | 2.52±0.56 | 0.37±0.10 | 3.49±0.36 |
| Low | 3.35±0.26 | 0.58±0.20** | 3.07±0.67 | 3.10±0.81 | 0.69±0.31** | 3.17±0.61 |
| Middle | 3.34±1.00 | 3.96±1.48** | 3.21±0.56 | 2.78±0.45* | 2.94±0.74*## | 3.30±0.59 |
| High | 5.93±1.68* | 1.20±0.51** | 3.08±1.10 | 3.97±0.54# | 1.03±0.35**# | 3.39±0.76 |
Suppression Effect of tetramethylpyrazine treatment on transcription factors (NF-κB, AP-1 and NF-AT) in SMC, LPMC, and PBMC
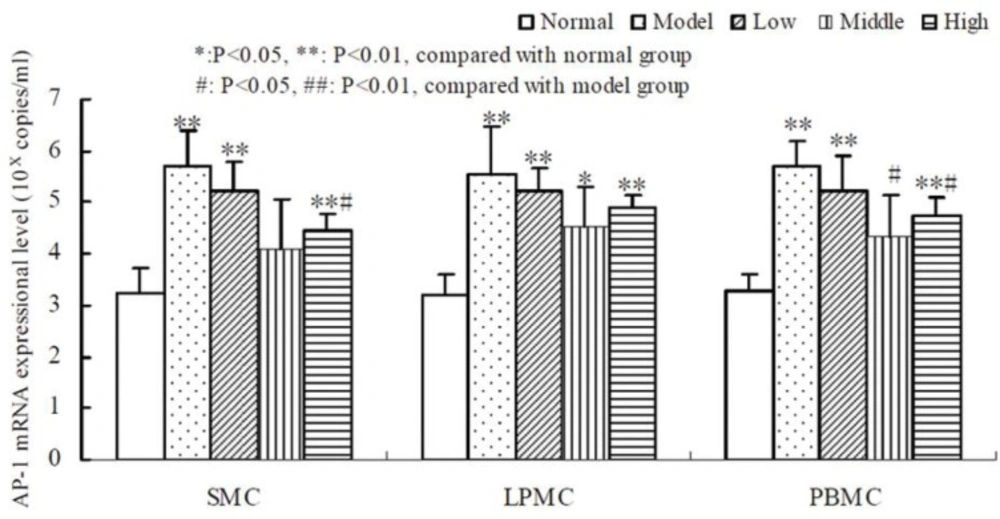
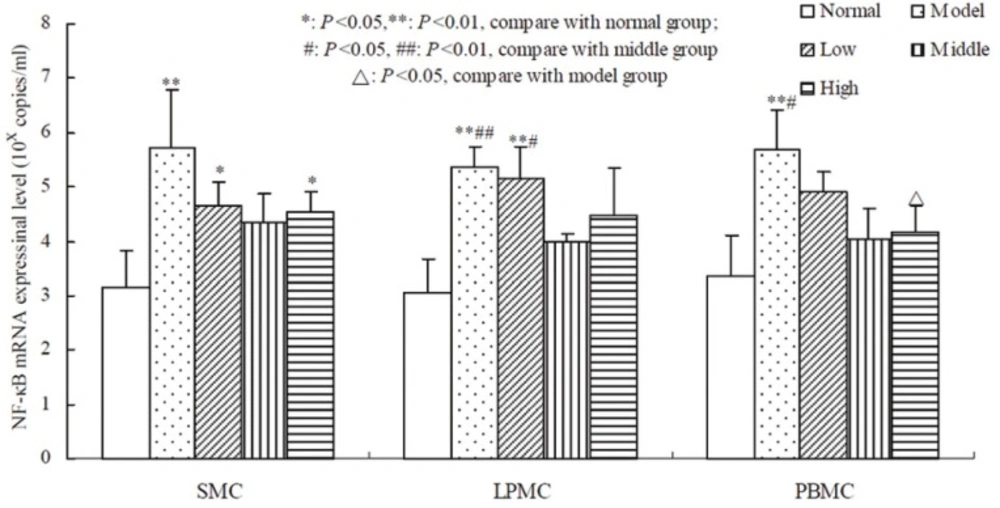
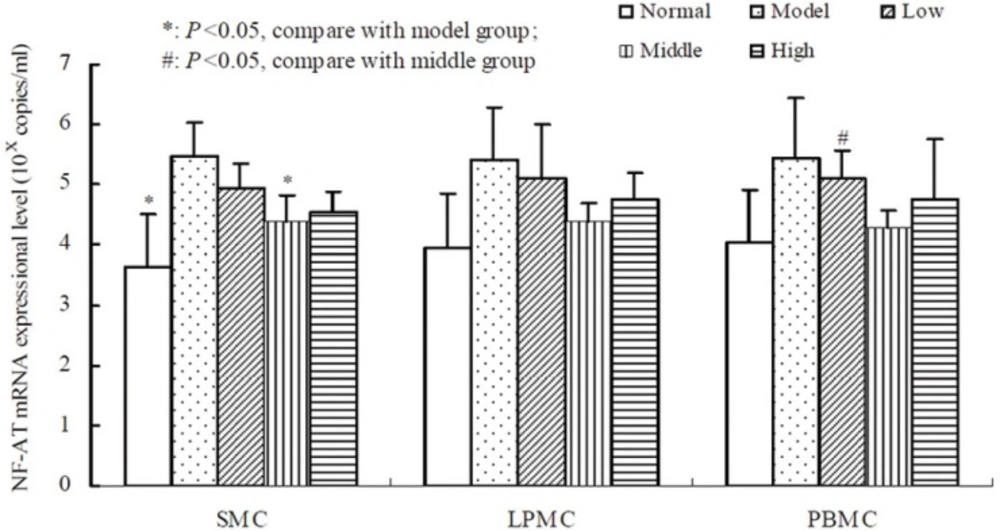
Compared to the normal group, oxazolone-induced colitis significantly promoted the expression of AP-1,NF-AT and NF-κB mRNA in SMC, LPMC, PBMC (P < 0.01, Table 3, 4, 5). The result showed that there was a non-linear relationship between the dose of tetramethylpyrazine and the expressional level of AP-1, NF-AT, and NF-κB (Figure 2, 3, 4). Compared with the other groups, AP-1, NF-AT, and NF-κB were most dramatically suppressed in SMC, LPMC and PBMC by the tetramethylprazine treatment in the middle dose group. However, there was significant difference in the mRNA level of AP-1 in SMC, LPMC and PBMC among the three different doses tetramethylpyrazine treated groups (P < 0.05). Moreover, no significant difference was observed in the mRNA levels of NF-AT and NF-κB in SMC, LPMC and PBMC between normal and middle groups.
: P < 0.05,
P < 0.01, compared with normal group.
: P < 0.05,
: P < 0.01, compared with model group.
: P < 0.05,
P < 0.01, compare with normal group;
: P < 0.05,
P < 0.01, compare with middle group
: P < 0.05,compare with model group
P < 0.05, compare with model group;
: P < 0.05, compare with middle group
RNA was extracted from the cells and qRT-PCR was performed to amplify AP-1 cDNA. The results showed that AP-1 mRNA level were much higher in SMC, LPMC and PBMC of the model group than those in normal group (P < 0.01). Dose-response studies showed that accompanying with a dose increase of tetramethylpyrazine, AP-1 mRNA were down-regulated compared with the model group, but still were higher than that in normal group. At a dose of 1 g/L (middle dose), AP-1 mRNA exhibited inhibition at the greatest degree.
RNA was extracted from the cells and qRT-PCR was performed to amplify NF-κB cDNA. The results showed that NF-κB mRNA level were significantly higher in SMC, LPMC and PBMC of the model group than those in normal group (P < 0.01). Dose-response studies showed that NF-κB mRNA of SMC in tetramethylpyrazine treated group at low dose and high dose was still higher than that in the normal group (P < 0.05). Only NF-κB mRNA of LPMC in the low dose group was still higher than that both in the normal group and middle dose group (P < 0.01, P < 0.05, respectively). NF-κB mRNA of PBMC in middle dose and high dose group was dramatically lower than that in the model group (P < 0.05).
RNA was extracted from the cells and qRT-PCR was performed to amplify NF-AT cDNA. NF-AT mRNA of SMC in model group was higher than that in both the normal group and the middle dose group (P < 0.05). There was no difference of NF-AT in LPMC among the groups. NF-κB mRNA of PBMC in middle dose was dramatically lower than that in low dose group (P < 0.05).
Tetramethylpyrazine (ligustrazine) is a kind of alkaloid extracted from Chinese traditional medicine, which can promote Qi-blood circulation and pain relief. It has been widely used in the treatment of spinal cord injury, cerebral ischemic injury (11) and tumor (12). A recent study reveals that expression of MyD88, interleukin (IL)-1, IL-23 and IL-6 in mononuclear phagocyte are required for colitis development (13). Oxazolone is a kind of half antigen which irritates contact allergies. Previous studies indicate that IL-4 is the initial cytokine produced in oxazolone-induced colitis and is rapidly superceded by IL-13 secreted by NK-T cells (3). In the present study, the effect of tetramethylpyrazine on mononuclear cells from spleen, lamina propria and peripheral blood in oxazolone-induced colitis were evaluated. We observed that mononuclear cells apoptosis in both spleen and lamina propria were reduced by oxazolone-induced progressive colitis. It indicated that both local intestine and the biggest immune organ-spleen were reacted with oxazolone. The apoptosis of SMC and LPMC was increased by tetramethylpyrazine treatment. The result was inconsistent with the previous studies which indicate that tetramethlpyrazine could reduce cell apoptosis in spinal cord via suppressing bcl-2 and caspase 3 (14, 15). The effect of tetramethylpyrazine on SMC and LPMC was related to dose in a nonlinear regression. The middle dose of tetramethylpyrazine had the most significant effect on the promotion of apoptosis for SMC and LPMC. It was indicated that the efficacy of tetramethlpyrazine was dependent on dose, but the proper therapeutic dose of tetramethlpyrazine for human UC need to be further investigated.
We also observed that the pathogenic pathway leading to tissue injury in oxazolone-induced colitis might be attributed to the production of cytokines. Cytokine expression is generally initiated and regulated at transcriptional level by interactions among specialized nuclear proteins, termed transcription factors and promoter region containing DNA elements that nucleotide sequences. Ap-1 is a dimeric complex of basic region-leucine zipper proteins, which consists of heterodimers or homodimers of Jun, Fos and ATF families. In a large number of genes, AP-1 binds to specific DNA sequences and regulates inflammation and cellular growth (-). NK-AT transcription factor is characterized by a highly conserved DNA binding domain and a calcineurin binding domain. Juan Y, et al. reported that tetramethylpyrazine plays an anti-inflammatory role, decreases IL-8 production in-vitro, and blocks ERK1/2 and p38 phosphorylation via suppressing AP-1 (19).
NF-AT factors play an important role in T cell activation and differentiation. Furthermore, NF-AT factors may also have an effect on the cycle and apoptosis of T lymphocytes. NF-κB, a pro-inflammatory transcription factor, has proved to be a key modulator governing the molecular network, and further leading to various cellular function abnormalities associated with IBD (20, 21). Compared with healthy control, intestinal tissues from IBD patients have enhanced NF-kB transcriptional activity.
Our data showed that AP-1, NF-AT and NF-kB mRNA in SMC, LPMC and PBMC were higher in the oxazolone-induced model group than those in the normal group. The suppression of AP-1, NF-AT and NF-kB by tetramethlpyrazine might be the molecular mechanism of the reduced apoptosis of mononuclear cells. Our results provided the evidence that AP-1, NF-AT and NF-kB might be the central targets of tetramethlpyrazine with its potent anti-inflammatory activity in oxazolone-induced colitis mice.
Conclusion
As shown in our study, tetramethylpyrazine inhibited the increase of NF- kB, AP-1 and NF-AT mRNA, and also promoted the apoptotic rate of SMC and LPMC in oxazolone-induced colitis mice. In conclusion, tetramethylpyrazine have the anti-inflammatory effect on oxazolone-induced colitis mice in dose-dependent manner. However, the anti-inflammatory effect on human colitis needs to be further confirmed by experimental study. The findings from the study might provide useful guidelines for clinical treatment of colitis.