Introduction
Verbascum genus is a member of Scrophulariaceae family and the genus is represented by 271 taxa in which 209 are endemic to Turkey (1-4). V. pinetorum (Boiss.) O. Kuntze is 40-100 cm and biannual plant. V. pinetorum is endemic to Turkey and is distributed in the East Mediterranean phytogeographical region. It is known in Adana, Hatay and Kahramanmaraş provinces. V. pinetorum grows in Pinus brutia forest and Quercus scrub and altitude between 370-1000 m. Flowering and fructification is between July and August (3). Vernacular name of V. pinetorum is Gavurdedengili.
Verbascum leaves and flowers are used as mucolytic, expectorant, diuretic and demulcent in traditional Turkish medicine and used to treat respiratory diseases such as tuberculosis, bronchitis, dry coughs, and asthma (5). Verbascum species are used as anti-inflammatory on urinary system; and as mild sedatives, and also used for treating rheumatic pain, hemorrhoids, wounds, diarrhea and fungal infections. Verbascum species consumed as a tea to relieve abdominal pains (6). Verbascum plants have been used for centuries in the folk’s medicine due to their wide spectrum of biological activities. Antimicrobial, antiviral and cytotoxic activities of some Verbascum species have been reported elsewhere (7). Some of Verbascum species are also used for liquor production (8).
Iridoids and its glycosides (9, 10), flavonoids (11), phenylethanoid and its glycosides (9,11, 12) and neolignan glycosides (12), and saponins (13, 14) have been isolated from Verbascum species and some of these compounds have anti-infectious properties and they have antioxidative, antiinflammatory, antitumor and immunostimulatory activities (15).
Synthetic antioxidants are used to extend the shelf life of foods and prevent degradation. However; there are many studies demonstrating the synthetic antioxidants and by-products formed of them can lead to various diseases (16, 17). For this reason, researches about new antioxidant substances in replace of synthetic ones have gained importance in this field. The antioxidants may be also relevant in slowing down the progression of Alzheimer’s disease. So, consumers who want to protect their health have started to deal with natural antioxidants more (18, 19).
Literature survey showed that there have been no previous reports about phytochemical profile with UHPLC-ESI-MS/MS, fatty acid composition on Verbascum species and ABTS cation radical decolorisation activity, cupric reducing antioxidant capacity, anticholinesterase activity and DNA damage effect of V. pinetorum. Aim of this study was to determine phytochemical profile, fatty acid and essential oil compositions, antioxidant, antialzheimer, antimicrobial activities and DNA cleavage protection of the extracts of endemic V. pinetorum from Turkey. This study is the first phytochemical and biological (ABTS, Cuprac, anticholinesterase and DNA damage protection effect) report on V. pinetorum.
Experimental
General experimental procedures
Phytochemical profile, essential oil and fatty acid compositions were determined by using Shimadzu UHPLC ESI MS/MS and GC/MS instruments, respectively. A Thermo pH-meter, Gel documentation System (Gel-Doc-XR, BioRad, Hercules, CA, USA), an Elma S15 ultrasonic bath,Horizontal electrophoresis (Biorad), Horizontal electrophoresis power supply (Wealtec), Shimadzu UV Spectrophotometer, a BioTek Power Wave XS and a vortex (LMS Co. LTD) were used for the activity assays. Ethanol, hexane, diethyl ether, chloroform, toluene, dichloromethane, methanol, potassium acetate, BHT (butylated hydroxytoluene) (purity ≥99%), sulphuric acid, aluminium nitrate nonahydrate, aluminium chloride, ABTS (2,2′-Azinobis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt) (97.5%), K2S2O8, sodium acetate, nutrient broth, boric acid, nutrient agar, NaHCO3 were purchased from Merck (Germany), (L)-malic acid (95-100%), quercetin (95%), protocatechuic acid (97%), chrysin (97%), rutin (94%), hesperetin (95%), naringenin (95%), rosmarinic acid (96%), vanillin (99%), p-coumaric acid (98%), caffeic acid (98%), chlorogenic acid (95%), hyperoside (≥97%), myricetin (≥96%), coumarin (≥99%), kaempferol (≥97%), 2,2-diphenyl-1-picrylhydrazyl (DPPH) (≥95%), β-carotene (≥93%), linoleic acid (≥99%), H2O2, Tween 40, pyrocathecol (≥99%), acetic acid, sodium methoxide, gel loading dye, DTNB (5,5-dithiobis-(2-nitro benzoic acid)) (≥98%), copper (II) chloride dihydrate (CuCl2.2H2O) (≥99%), neocuproine (2,9-dimethyl-1,10-phenanthroline) (≥98%), EDTA (≥98%), acetylcholinesterase, butyrylcholinesterase, trisma base, galanthamine hydrobromide (≥94%) from Sigma (Germany), α-tocopherol (≥95.5%), acetylthiocholine iodide (≥98%) from Aldrich (Germany), quinic acid (98%), tr-aconitic acid (98%), 4-hydroxybenzoic acid (≥99%) and fisetin (≥98%) were from Aldrich (Germany), gallic acid (≥99%), tannic acid (puris), salicylic acid (≥99%) were from Sigma-Aldrich (Germany), Folin Ciocalteu Phenol reagent from Applichem (Germany), hesperidin (≥97%), luteolin (≥97%), apigenin (≥99%), rhamnetin (≥99%), butyrylthiocholine iodide (≥99%) from Fluka (Germany), steril blank disc and antbiotic disc from Oxoid (United Kingdom), acetone, petroleum ether, sodium carbonate, sodium dihydrogen phosphate, sodium hydrogen phosphate, ammonium acetate from Reidel de Haen (Germany).
Plant material
S. Demirci collected and identified the plant material from southern Turkey (Andırın, Kahramanmaraş) in June 2012. A sample was deposited in the Herbarium of Istanbul University (ISTE 97137).
Preparation of plant extracts for UHPLC-ESI-MS/MS
The dried and powdered plants (10 g) were extracted separately with MeOH and acetone about 24 h at room temperature. The extracts were filtrated and evaporated under vacuum. Dry filtrates diluted until 250 mg/L and passed through 0.2 µM microfiber filter for UHPLC-ESI-MS/MS.
Instruments and Chromatographic Conditions
LC-MS/MS analyses of the twenty seven compounds were performed by using a Nexera model Shimadzu UHPLC coupled to a tandem MS instrument. The liquid chromatograph was equipped with LC-30AD binary pumps, DGU-20A3R degasser, CTO-10ASvp column oven and SIL-30AC autosampler. The chromatographic seperation was performed on a C18 reversed-phase Inertsil ODS-4 (150 mm × 4.6 mm, 3µM) analytical column. The column temperature was fixed at 40 ° C. The elution gradient consisted of mobile phase A (water, 5mM ammonium formate and 0.1% formic acid) and mobile phase B (methanol, 5mM ammonium formate and 0.1% formic acid). The gradient program with the following proportions of solvent B was applied t (min), %B: (0, 40), (20, 90), (23.99, 90), (24, 40), (29, 40). The solvent flow rate was maintained at 0.5 mL/min and injection volume was settled as 4 µL.
MS Instrumentation
MS detection was performed using Shimadzu LC-MS 8040 model triple quadrupole mass spectrometer equipped with an ESI source operating in both positive and negative ionization modes. LC-MS/MS data were collected and processed by LabSolutions software (Shimadzu, Kyoto, Japan). The multiple reaction monitoring (MRM) mode was used to quantify the analytes: the assay of investigated compounds was performed following two or three transitions per compound, the first one for quantitative purposes and the second and/or the third one for confirmation.
Optimization of UHPLC-MS/MS Method
Subsequent to several combinations of trials, a gradient of methanol (5 mM ammonium formate and 0.1% formic acid) and water (5mM ammonium formate and 0.1% formic acid) system was concluded to be the best mobile phase solution. For rich ionization and the seperation of the molecules, the mentioned mobile phase was proved to be the best of all. ESI source was chosen instead of APCI (Atmospheric Pressure Chemical Ionization) and APPI (Atmospheric Pressure Photoionization) sources as the phenolic compounds were small and relatively polar molecules. Tandem mass spectrometry was decided to be used for the current study since this system is commonly used for its fragmented ion stability. The working conditions were determined as interface temperature; 350 ° C, DL temperature; 250 ° C, heat block temperature; 400 ° C, nebulizing gas flow (Nitrogen); 3 L/min and drying gas flow (Nitrogen); 15 L/min.
Esterification of total fatty acids and GC/MS conditions
Esterification of petroleum ether extract of V. pinetorum was done according to the report of Ertaş et al. (19). In this study, Thermo Scientific Polaris Q GC-MS/MS was used. GC-MS study conditions, identification and quantification of the compounds comparison were done exactly same manner according to Ertaş et al. (19).
Isolation of essential oil and GC/MS conditions
Clevenger-type apparatus was used for obtaining essential oil of V. pinetorum. The essential oil was diluted for the GC process by dichloromethane (1:3, v/v). GC/MS analyses were performed on Thermo Electron Trace 2000 GC model gas chromatography and Thermo Electron DSQ quadrupole mass spectrometry. A nonpolar Phenomenex DB5 fused silica column (30 m´ 0.32 mm, 0.25 μM film thickness) was used with helium at 1mL/min (20 psi) as a carrier gas. The GC oven temperature was kept at 60 ° C for 10 min and programmed to 280 ° C for 10 min. The split ratio was adjusted to 1:50, the injection volume was 0.1 μL and EI/MS was recorded at 70eV ionization energy. Mass range was m/z35-500 amu. Identification of the compounds was based on the comparison of their retention times and mass spectra with those obtained from authentic samples and/or the NIST and Wiley spectra as well as the literature data.
Preparation of the extracts
Whole parts of V. pinetorum (100 g) were dried under shadow and powdered, and then they were sequentially macerated 3 times with petroleum ether, acetone, methanol and water (250 mL) for 24 h at room temperature, respectively. After filtration, the solvents were evaporated to obtain crude extracts. The yield of the extracts are petroleum ether extract 0.60%, acetone extract 1.20%, methanol extract 5.00% and water extract 2.30% (w/w).
Determination of total phenolic and flavonoid contents of the extracts
The amounts of phenolic and flavonoid contents in the crude extracts were expressed as pyrocatechol and quercetin equivalents, and they were calculated according to the following equations (20, 21):
Absorbance = 0.0125 pyrocatechol (μg) + 0.0347 (R2 = 0.9928)
Absorbance = 0.0301 quercetin (μg) + 0.0553 (R2 = 0.9984)
Antioxidant activity of the extracts
β-Carotene-linoleic acid test system (22), DPPH free radical scavenging activity (23), ABTS cation radical decolorisation (24) and cupric reducing antioxidant capacity (CUPRAC) (25) methods were carried out to determine the antioxidant activity.
Anticholinesterase activity of the extracts
A spectrophotometric method developed by Ellman et al. was established to indicate the acetyl- and butyryl-cholinesterase inhibitory activities (26).
Determination of antimicrobial activity and Minimum Inhibitory concentration (MIC)
Five different microorganisms including gram positive bacteria (Streptococcus pyogenes ATCC19615 and Staphylococcus aureus ATCC 25923), gram negative bacteria (Pseudomonas aeruginosa ATCC 27853, Escherichia coli ATCC 25922) and yeast (Candida albicans ATCC10231) which were purchased from Refik Saydam Sanitation Center (Turkey) were used for detecting the antimicrobial activity of the samples. The disc diffusion method was employed for this purpose (27, 28). The minimum inhibitory concentration determined by the broth macrodilution method according to NCCLS (29). Ampicillin and fluconazole were used as positive controls for bacteria and yeast, respectively.
DNA damage protective activity of the extracts
Measurement of the DNA damage protective activity of the methanol extract was checked on pBluescript M13(+) plasmid DNA. Plasmid DNA was oxidized with OH radicals which generated from UV photholysis of H2O2 in the presence of theextract and checked on 1% agarose according to Kızıl et al. (30). Percent inhibition of the DNA cleavage protection was calculated using the method described by Fukuhara et al. (31).
Statistical analysis
The results of the antioxidant, anticholinesterase and antimicrobial activity assays were mean ± SD of three parallel measurements. The statistical significance was estimated using a Student’s t-test, p values <0.05 were regarded as significant.
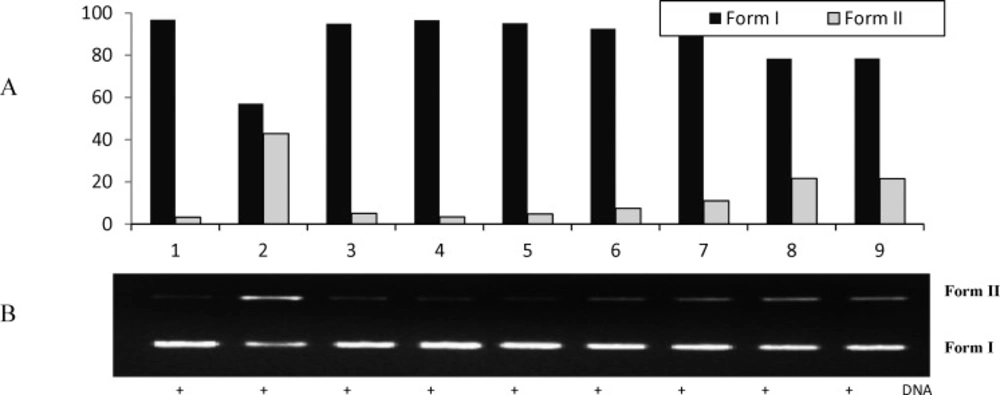
The quantified band intensity for the scDNA (Form I), ocDNA (Form II) with Quantity One 4.5.2. version software (A). Electrophoretic pattern of pBluescript M13+ DNA after UV-photolysis of H2O2 in the presence or absence of V. pinetorum methanol extract. Reaction vials contained 200 ng of supercoiled DNA (31.53 nM) in distilled water, pH 7). Electrophoresis was performed using 1% agarose at 40V for 3 h in the presence of ethidium bromide (10 mg/mL) (B). Electrophoresis running buffer: TAE (40 mM Tris acetate, 1 mM EDTA, pH 8.2). Gel was scanned on Gel documentation system (Gel-Doc-XR, BioRad, Hercules, CA, USA). Bands on the gels were quantified using discovery series Quantity One programme (version 4.5.2. BioRad Co
| No | Analyte | Parent ion (m/z)a | MS2(CE)b | Ionization Mode | RTc | R2,d | RSD%e | Linearity Range (mg/L) | LOD/LOQ (µg/L)f | Recovery (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Quinic acid | 190,95 | 85 (22),93 (22) | Neg | 3.32 | 0.9927 | 0.0388 | 250-10000 | 22.3 / 74.5 | 103.3 |
| 2 | Malic acid | 133,05 | 115 (14),71 (17) | Neg | 3.54 | 0.9975 | 0.1214 | 250-10000 | 19.2 / 64.1 | 101.4 |
| 3 | tr-Aconitic acid | 172,85 | 85 (12),129 (9) | Neg | 4.13 | 0.9933 | 0.3908 | 250-10000 | 15.6 / 51.9 | 102.8 |
| 4 | Gallic acid | 169,05 | 125 (14),79 (25) | Neg | 4.29 | 0.9901 | 0.4734 | 25-1000 | 4.8 / 15.9 | 102.3 |
| 5 | Chlorogenic acid | 353 | 191 (17) | Neg | 5.43 | 0.9932 | 0.1882 | 250-10000 | 7.3 / 24.3 | 99.7 |
| 6 | Protocatechuic acid | 152,95 | 109 (16),108 (26) | Neg | 5.63 | 0.9991 | 0.5958 | 100-4000 | 25.8 / 85.9 | 100.2 |
| 7 | Tannic acid | 182,95 | 124 (22),78 (34) | Neg | 6.46 | 0.9955 | 0.9075 | 100-4000 | 10.2 / 34.2 | 97.8 |
| 8 | tr- caffeic acid | 178,95 | 135 (15),134 (24),89 (31) | Neg | 7.37 | 0.9942 | 1.0080 | 25-1000 | 4.4 / 14.7 | 98.6 |
| 9 | Vanillin | 151,05 | 136 (17),92 (21) | Neg | 8.77 | 0.9995 | 0.4094 | 250-10000 | 10.1 / 33.7 | 99.2 |
| 10 | p-Coumaric acid | 162,95 | 119 (15),93 (31) | Neg | 9.53 | 0.9909 | 1.1358 | 100-4000 | 15.2 / 50.8 | 98.4 |
| 11 | Rosmarinic acid | 358,9 | 161 (17),133 (42) | Neg | 9.57 | 0.9992 | 0.5220 | 250-10000 | 10.4 / 34.8 | 101.7 |
| 12 | Rutin | 609,1 | 300 (37), 271 (51), 301 (38) | Neg | 10.18 | 0.9971 | 0.8146 | 250-10000 | 17.0 / 56.6 | 102.2 |
| 13 | Hesperidin | 611,1 | 303 (24),465 (12) | Poz | 9.69 | 0.9973 | 0.1363 | 250-10000 | 21.6 / 71.9 | 100.2 |
| 14 | Hyperoside | 463,1 | 300 (27),301 (26) | Neg | 10.43 | 0.9549 | 0.2135 | 100-4000 | 12.4 / 41.4 | 98.5 |
| 15 | 4-OH Benzoic acid | 136,95 | 93 (17),65 (27) | Neg | 11.72 | 0.9925 | 1.4013 | 25-1000 | 3.0 / 10.0 | 106.2 |
| 16 | Salicylic acid | 136,95 | 93 (16),65 (31),75 (30) | Neg | 11.72 | 0.9904 | 0.6619 | 25-1000 | 4 / 13.3 | 106.2 |
| 17 | Myricetin | 317 | 179 (19),151 (23),137 (26) | Neg | 11.94 | 0.9991 | 2.8247 | 100-4000 | 9.9 / 32.9 | 106.0 |
| 18 | Fisetin | 284,95 | 135 (22),121 (27) | Neg | 12.61 | 0.9988 | 2.4262 | 100-4000 | 10.7 / 35.6 | 96.9 |
| 19 | Coumarin | 146,95 | 103 (17),91 (26),77 (27) | Poz | 12.52 | 0.9924 | 0.4203 | 100-4000 | 9.1 / 30.4 | 104.4 |
| 20 | Quercetin | 300,9 | 179 (19),151 (21),121 (28) | Neg | 14.48 | 0.9995 | 4.3149 | 25-1000 | 2.0 / 6.8 | 98.9 |
| 21 | Naringenin | 270,95 | 151 (18),119 (24),107 (26) | Neg | 14.66 | 0.9956 | 2.0200 | 25-1000 | 2.6 / 8.8 | 97.0 |
| 22 | Hesperetin | 300,95 | 164 (25),136 (33),108 (42) | Neg | 15.29 | 0.9961 | 1.0164 | 25-1000 | 3.3/ 11.0 | 102.4 |
| 23 | Luteolin | 284,95 | 217 (25),199 (28),175 (29),151 (25) | Neg | 15.43 | 0.9992 | 3.9487 | 25-1000 | 5.8 / 19.4 | 105.4 |
| 24 | Kaempferol | 284,95 | 217 (29),133 (32),151 (23) | Neg | 15.43 | 0.9917 | 0.5885 | 25-1000 | 2.0 / 6.6 | 99.1 |
| 25 | Apigenin | 268,95 | 151 (25),117 (35) | Neg | 17.31 | 0.9954 | 0.6782 | 25-1000 | 0.1 / 0.3 | 98.9 |
| 26 | Rhamnetin | 314,95 | 165 (23),121 (28),300 (22) | Neg | 18.94 | 0.9994 | 2.5678 | 25-1000 | 0.2 / 0.7 | 100.8 |
| 27 | Chrysin | 253 | 143 (29),119 (32),107 (26) | Neg | 21.18 | 0.9965 | 1.5530 | 25-1000 | 0.05 / 0.17 | 102.2 |
Parent ion (m/z): Molecular ions of the standard compounds (mass to charge ratio)
MS2(CE): MRM fragments for the related molecular ions (CE refers to related collision energies of the fragment ions)
RT: Retention time
R2: coefficient of determination
R2: coefficient of determination
LOD/LOQ (µg/L): Limit of deteection/Limit of quantification
U (%): Percent relative uncertainty at 95% confidence level (k=2).
Values in µg/g (w/w) of plant extract
N.D: not detected
Retention time (as minutes)
A nonpolar Phenomenex DB-5 fused silica colum
| Rt (min)a | Constituentsb | % Composition | RIc |
|---|---|---|---|
| 10.87 | Isononane | 2.6 | 865 |
| 15.20 | β-pinene | 2.3 | 979 |
| 17.15 | Cineole | 16.9 | 1031 |
| 24.06 | 1,3-Di-tert butyl benzene | 3.2 | 1249 |
| 25.80 | Dihydro carvyl acetate | 3.5 | 1344 |
| 30.30 | τ-Muurolene | 3.4 | 1480 |
| 30.48 | Valencene | 2.7 | 1484 |
| 30.87 | α-Selinene | 16.4 | 1498 |
| 35.52 | 2-Methyl heptadecane | 2.8 | 1746 |
| 36.45 | Octadecane | 2.9 | 1800 |
| 36.74 | 2-Methyl-1-hexadecanol | 2.4 | 1890 |
| 36.93 | 1-Nonadecanol | 2.8 | 2156 |
| 40.00 | Heneicosane | 3.1 | 2109 |
| 40.13 | 2,5-Di-tert octyl-p-benzoquinone | 7.8 | 2259 |
| 40.59 | Arachidic acid | 3.5 | 2366 |
| 40.66 | Hexadecanoic acid | 2.4 | 1986 |
| 40.84 | Tetracosane | 2.4 | 2407 |
| 41.13 | 3-Ethyl-5-(2-ethylbutyl)octadecane | 2.8 | 2413 |
| 43.30 | Heptacosane | 3.1 | 2700 |
| 43.84 | Choleic acid | 2.9 | 2896 |
| 44.41 | Ethyl iso-allocholate | 2.3 | 3094 |
| 45.11 | 17-pentatriacontene | 2.6 | 3508 |
| 46.50 | Hexatriacontane | 2.3 | 3600 |
| 47.12 | Tetratetracontane | 2.4 | 4400 |
| Total | 99.5 |
Retention time (as minutes).
A nonpolar Phenomenex DB-5 fused silica column
RI Retention indices (DB-5 column)
| Extracts | Phenolic content | Flavonoid content | Inhibition % | Inhibition % |
|---|---|---|---|---|
| VPP | 139.20 ± 2.83 | 92.09 ± 1.38 | NA | 44.02 ± 0.98 |
| VPA | 577.20 ± 2.63 | 111.03 ± 1.21 | NA | 15.64 ± 0.56 |
| VPM | 293.11 ± 1.31 | 27.97 ± 0.33 | NA | 25.05±0.19 |
| VPW | 339.42 ± 1.11 | 74.15 ± 0.23 | NA | 11.14±0.72 |
| Galanthaminey | - | - | 75.11 ± 0.69 | 82.49 ± 0.32 |
Values expressed are means ± S.D. of three parallel measurements, different letters in the same column indicate a significant difference (p< 0.05),
Standard drug, NA: Not active,
PEs, pyrocatechol equivalents (y = 0.0125 x + 0.0347 R2 = 0.9928),
QEs, quercetin equivalents (y = 0.0301 x – 0.0553 R2 = 0.9984).
| Microorganisms | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gram positive | Gram negative | Yeast | ||||||||||
| S. aureus | S.pyogenes | E.coli | P. aeruginosa | C. albicans | ||||||||
| Acetone extract | aDD | 8±0.6 | 10±0.2 | 10±0.3 | - | 16±0.2 | ||||||
| MIC | 85±0.2 | >1000 | >1000 | - | 25±0.3 | |||||||
| Methanol extract | aDD | 8±0.3 | 8±0.5 | 10±0.2 | 10±0.3 | - | ||||||
| MIC | 40±0.5 | >1000 | >1000 | >1000 | - | |||||||
| Water extract | aDD | 10±0.3 | 10±0.3 | NZ | 10±0.2 | - | ||||||
| MIC | 30±0.2 | >1000 | - | >1000 | - | |||||||
| Positive controls | bDD | 35±0.2 | 19±0.2 | 20±0.1 | - | 30±0.3 | ||||||
| MIC | 1.95±0.3 | 7.815±0.1 | 7.815±0.4 | - | 3.125±0.2 | |||||||
DD: Inhibition zone in diameter (mm) around the discs (6 mm) impregnated with 30 mg mL-1 of plant extracts.
DD: Inhibition zone in diameter (mm) of positive controls that are ampicillin for bacteria and fluconazole for yeast. Minimum inhibitory concentration (MIC) values are given as μg mL-1
Results and discussion
Quantitative analysis of phenolic compounds by UHPLC-ESI (QqQ)/MS/MS
Several studies are present in literature reporting the use of liquid chromatography electrospray ionization tandem mass spectrometry to perform quantitative analyses (32-34). Thus, for quantitative purpose, an accurate method on a mass spectrometer equipped with a triple quadrupole analyzer was developed for the analyses of twenty-seven compounds. Methanol and acetone extracts of V. pinetorum were analysed in order to quantify the twenty seven compounds.
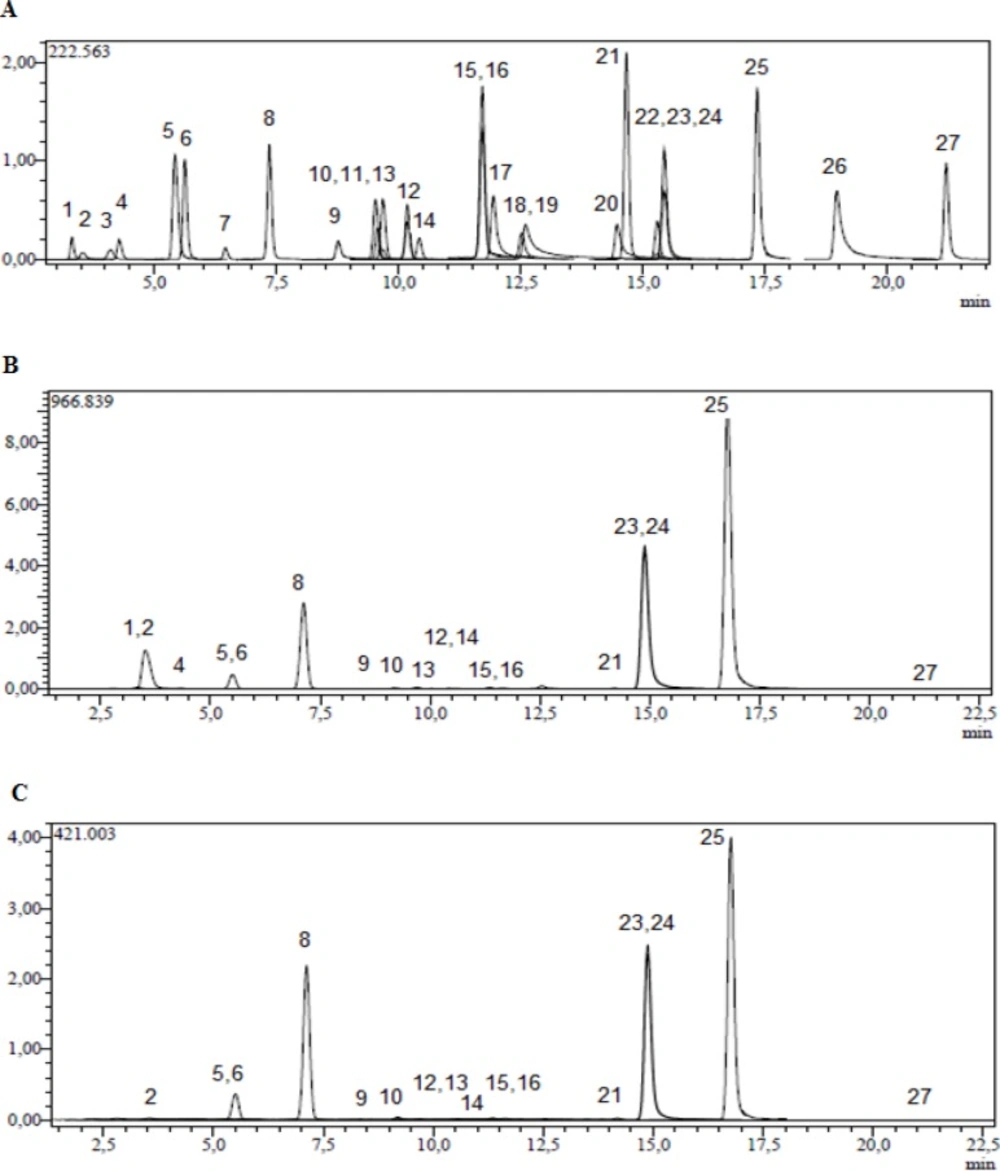
In order to monitor the mentioned compounds by MRM, the specific fragmentation reactions were selected. Twenty-seven compounds including (L)-malic acid, quercetin, protocatechuic acid, chrysin, rutin, hesperetin, naringenin, rosmarinic acid, vanillin, p-coumaric acid, caffeic acid, chlorogenic acid, hyperoside, myricetin, coumarin, kaempferol, quinic acid, tr-aconitic acid, 4-hydroxybenzoic acid, fisetin, gallic acid, tannic acid, salicylic acid, hesperidin, luteolin, apigenin, rhamnetin were monitored by the transition from the specific deprotonated molecular ions [M-H+] to the corresponding fragment ions [M-H+-X] and Figure 1A shows their chromatograms. Table 1. shows molecular ions, fragments observed in MS/MS, related collision energies for these fragments and the quantified results for methanol and acetone extracts of V. pinetorum.
For metanol and acetone extract of V. pinetorum; tr-aconitic acid, tannic acid, rosmarinic acid, myricetin, fisetin, coumarin, quercetin, hesperetin, rhamnetin compounds and for acetone extract of V. pinetorum; quinic acid, gallic acid were not detected and quantified by this method. In the methanol extract of V. pinetorum, malic acid (47250.61 µg/g), apigenin (16875.45 µg/g), kaempferol (16834.42 µg/g) and luteolin (15693.14 µg/g) were found to be major compounds (Table 1, Figure 1B) and for acetone extract of V. pinetorum, luteolin (7651.96 µg/g), kaempferol (7552.63 µg/g) and apigenin (7159.58 µg/g) were the most abundant compounds (Table 1., Figure 1C.). In literature several phenolic compounds such as chlorogenic acid, caffeic acid, ferulic acid, rosmarinic acid, quercetin, apigenin and aucubin from V. phlomoides (35), luteolin, apigenin, diosmin and 7-glucosides of luteolin, apigenin and quercetin from V. densiflorum and V. phlomoides (11), caffeic, syringic, p-coumaric, ferulic acid, quercetin and rutin from V. pestalozzae (36), protocatechuic, chlorogenic, vanillic, p-coumaric acid and rutin from V. detersile (36), protocatechuic, chlorogenic, p-coumaric acid, quercetin and rutin from V. bellum (36), protocatechuic, chlorogenic, caffeic, p-coumaric, ferulic acid and rutin from V. myriocarpum (36) have been detected in Verbascum species by HPLC DAD technique. In literature, there is not any study about qualitative and quantitative phenolic and flavonoid constituents on Verbascum species with UHPLC-MS/MS instrument.
There is no report about fatty acid composition on Verbascum species and also essential oil composition studies on V. pinetorum. This is the first study which report fatty acid and essential oil composition of V. pinetorum. As shown in Table 2. fatty acid composition of petroleum ether extract of V. pinetorum was determined by GC and GC-MS analysis and 9 components were identified, constituting 99.8%. The main components of fatty acid were found to be palmitic (27.1%), stearic (22.1%) and linoleic acids (17.1%). In the study of Yılmaz-İskender et al. (37) flower, leaf and stem of V. wiedemannianum were analysed for their volatile components. The main group of contituents of oil composition of flower and stem is hydrocarbons with 83.3% and 32.1% ratios, respectively. Major group of leaf oil was found to be aldehydes with 46.8% ratio. Main components of flower, leaf and stem oils were pentadecane (58.2%), (2E)-hexanal (33.2%) and hexadecanoic acid (24.6%), respectively.
There is no report about essential oil composition of V. pinetorum. The essential oil composition of V. pinetorum were determined by GC and GC-MS analysis. 24 compounds were identified, constituting 99.5% of total oil (Table 3.). The main compounds of the essential oil were cineole (16.9%) and α-selinene (16.4%). According to the report of Melliou et al. (38) thirty-six compounds were identified from essential oil of V. undulatum Lam and the main compounds were 1-octen-3-ol (22.5%) and α-bisabolol (10.6%). The antioxidant activity of the petroleum ether (VPP), acetone (VPA), methanol (VPM) and water (VPW) extracts prepared from the whole plant of V. pinetorum was investigated by using β-carotene bleaching, DPPH free radical scavenging, ABTS cation radical decolorisation and cupric reducing antioxidant capacity assays with their total phenolic and flavonoid contents.
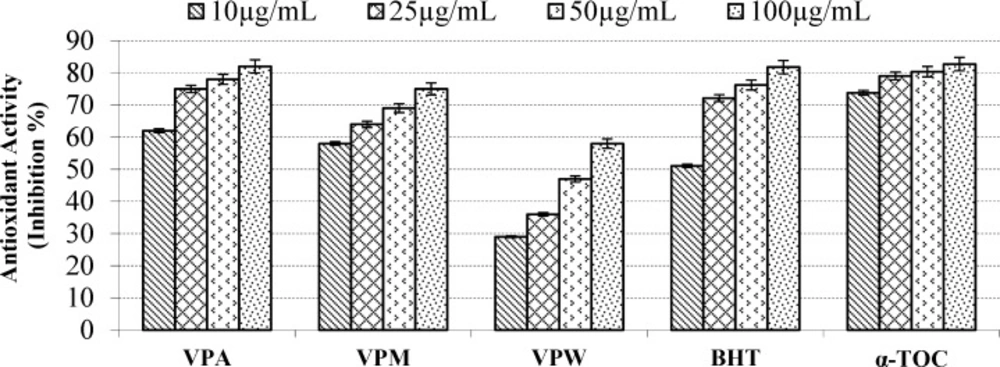
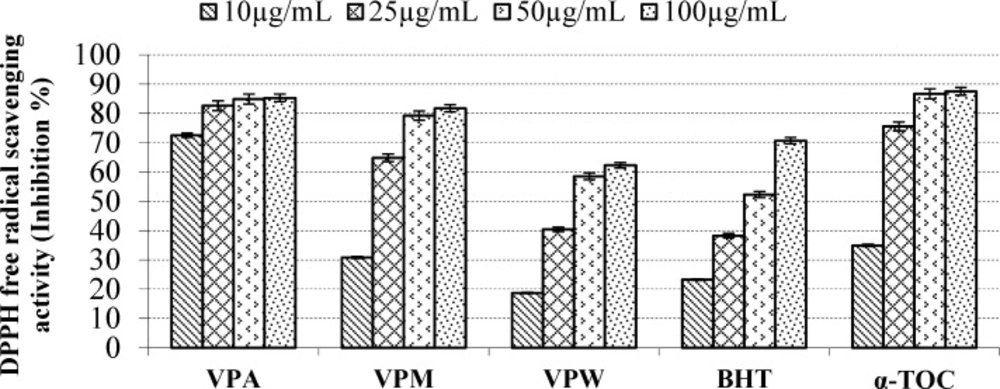
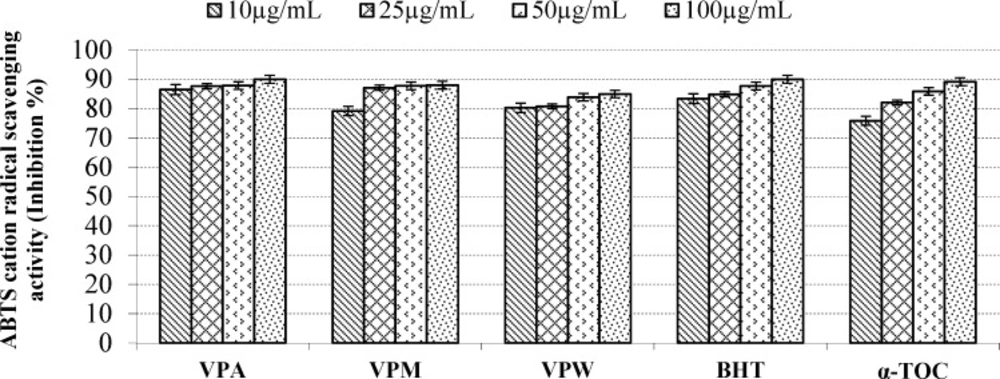
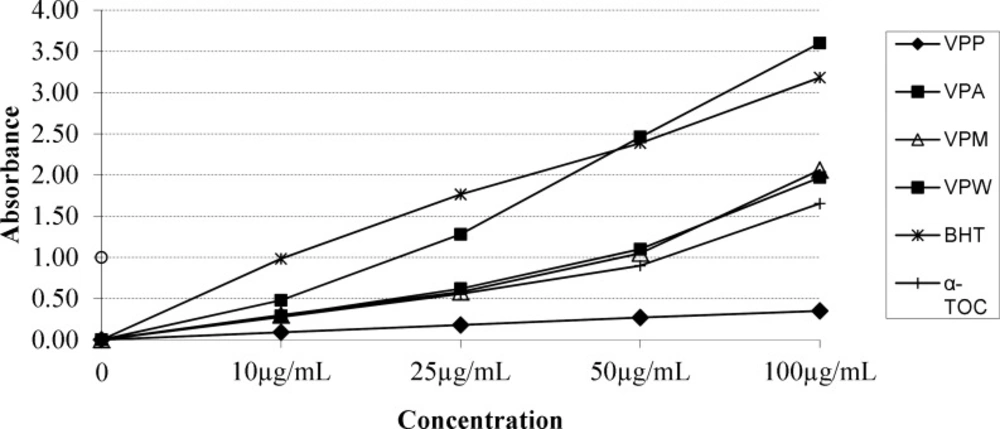
According to report of Alan et al. (39) on the antioxidant activities of three Verbascum species, employing different extraction solvents, the methanol and water extracts exerted greater antioxidant activity than other extracts. Results of the study of Saltan et al. (36) indicate that the methanol extract of various Verbascum species showed good antioxidant acitivty in β-carotene-linoleic acid test system and DPPH free radical scavenging assays. In β-carotene-linoleic acid test system, the acetone extract of V. pinetorum showed better inhibition than BHT at all concentrations (Figure 2.) in present study. The acetone and methanol extracts of V. pinetorum showed strong inhibition in DPPH free radical scavenging and have found to be higher than BHT at all concentration (Figure 3.). The acetone, methanol and water extracts of V. pinetorum exhibited significant inhibition in ABTS cation radical scavenging assay and also the acetone extract of V. pinetorum is more active than BHT and α-Toc used as standards (Figure 4.). In CUPRAC reducing antioxidant capacity assay, the acetone, methanol and water extracts showed strong activity; while the activity of the acetone extract was even better than standards BHT and α-Toc. Also the methanol and water extracts showed higher activity than the standard α-Toc (Figure 5.). As seen in Table 4., total phenolic and flavonoid contens of acetone extract were the richest and total phenolic content is more than total flavonoid content for all extracts. There is no activity against acethylcholinesterase and weak activity against butyrylcholinesterase extracts of V. pinetorum in antialzheimer activity assays (Table 4.).
The antimicrobial activities of the extracts against different microorganisms were assessed according to inhibition zone diameter and MIC value. Results are presented in Table 5. In reports of Saltan et al. (36), generally ethylacetate extracts were effective for E. coli. Ethylacetate extract of V. pestalozzae showed the best inhibitory effect on P. aeroginosa. The antimicrobial activity of V. pinetorum.extracts against different microorganisms were assessed according to inhibition zone diameter. Among the extracts only the petroleum ether extract was not active on microorganisms (data not shown). The acetone, methanol and water extracts showed activity in different degrees. The acetone extract showed weak antimicrobial activity (inhibition zone < 12) against E. coli, S. pyogenes, S. aureus; moderate activity (inhibition zone < 20-12) against C. albicans; and no activity against P. aeruginosa. The methanol extract showed weak antimicrobial activity against bacteria and no activity against the yeast. The water extract was active only on P. aeruginosa, S. pyogenes and S. aureus. The lowest MIC value was recorded by acetone extract against C. albicans (25 µg mL-1).
The inhibition activities of the methanol extract of V. pinetorum on DNA damage were found to be 89.34, 80.31, 53.91 and 53.68 % at the concentrations of 100, 250, 350 and 500 μg/mL, respectively. It can be seen in lane 5 that only the methanol extract V. pinetorum (250 μg) has no significant effect on DNA cleavage protection (Figure 6.).
Conclusions
Present study showed that the acetone and methanol extracts of V. pinetorum shows strong antioxidant activity in the all activity assays namely β-carotene-linoleic acid test system, DPPH free radical scavenging, ABTS cation radical decolorisation and cupric reducing antioxidant capacity methods. Results reported in this study can be considered as the first detailed study on the phytochemical content and in-vitro antioxidant, anticholinesterase, antimicrobial activities and DNA damage protection effect of V. pinetorum. Further investigation should be done for isolation and identification of antioxidant active constituents of the extracts, especially acetone and methanol extract.