Introduction
Staphylococcus aureus is a major common cause of skin and soft tissue infections, although the majority of serious infections occur in the health care setting and in hospitals (1). Treatment of severe S. aureus infections can be puzzling and mortality rate remains between 20-25% (2). Over the past 40 years, methicillin resistant S. aureus had become widespread in worldwide hospitals (3). Increasing incidence of antibiotic resistance poses a serious risk to human health (4). The invasion of host tissues by S. aureus αβγδβ5). Approximately 70 different extra-cellular proteins were detected in strain N315 using a proteomics approach (6) but some expected proteins were not detected e.g. nuclease. Over 100 extracellular proteins were detected by Royan et al., (7). Many of these exoproteins have been shown to play a direct role in the pathogenicity of the organism or to enhance virulence (5-8).
Silver sulphadiazine (AgSD) is very effective for prevention and treatment of infection in burn wounds which is widely used (9-11). As it is likely that at least some areas of treated wounds will be exposed to low concentrations we have determined the effects of sub-inhibitory concentrations on toxin synthesis and in previous reports we showed that the production of Toxic Shock Syndrome Toxin-1 and exoproteases were affected by sub-inhibitory concentrations of silver sulphadiazine (12, 13). Silver sulphadiazine (AgSD) increased levels of TSST-1 in strains T4 and RN4282 (termed responder strains) but no changes in TSST-1 were seen in strain T1 (termed a non-responder strain). AgSD also repressed metalloprotease production in strain T4 and stimulated thiol-protease so that the overall protease activity remained the same. There were no effects on proteolytic activities in strain T1. In order to further define the effects of AgSD in this study we investigated the effects of AgSD on extracellular protein production in a number of responder and non-responder strains isolated from burn wounds.
Experimental
Bacterial strains and culture conditions
Eleven strains of S. aureus were used for this study (Table 1). T1 (FRI1189S) was originally isolated from a case of menstrual toxic shock syndrome (MTSS) and supplied by M. S. Bergdoll; T4 was from a burned patient with confirmed non-menstrual toxic shock syndrome (NMTSS, Bury and Rochdale Healthcare Trust, UK), RN4282 was obtained from T.J. Foster, University of Dublin, Ireland and 8325-4 from R. Novick, New York University of Medicine, USA. All other strains were isolated from burn wounds (12). S. aureus RN4282, T4, B6, B23, B45 and B54 were responders, T1, B3 and B35 were non-responders, 8325-4 had not been tested and B10 did not produce TSST-1 and was included as a control. All strains were confirmed as S. aureus by performing Gram stain, catalase, oxidase and Staphaurex (Murex Biotech Limited, Kent, UK) tests.
| Strain | Gender/Age | Isolation | Toxin | Sapi | Responder |
|---|---|---|---|---|---|
| T1 | unknown | MTSS | TSST-1 | SAPI2 | NR |
| T4 | unknown | NMTSS | TSST-1 | SAPI2 | R |
| B3 | M/3y | Right arm | TSST-1 | SAPI2 | R |
| B6 | M/4y | Hand | TSST-1 | SAPI2 | NR |
| B10 | F/10m | Chest | Ent C | NT | |
| B23 | M/unknown | Chest | TSST-1/ Ent A | SAPI2 | R |
| B35 | M/11y | Skin | TSST-1 | SAPI2 | NR |
| B45 | M/11y | Abdomen | TSST-1 | SAPI1 | R |
| B54 | F/unknown | unknown | TSST-1 | SAPI1 | R |
| 8325-4 | Laboratory strain | TSST-1 | SAPI1 | NT | |
Kinetics of exoprotein production in sub-inhibitory concentrations of silver sulphadiazine
Overnight broth cultures of S. aureusμ-1 of AgSD and incubated at 37 °C as before. Flasks were removed at 2, 4, 8, 24, 48 and 72 h, turbidities were determined using a Unicam SP600 spectrophotometer at 550 nm using distilled water as a blank. Cultures with optical densities above 0.7 were diluted 1:10 with distilled water. The remainder was centrifuged for 20 min at 1500 x g and 4 °C and the supernatant fluids removed and stored at –20 °C.
Preparation of protein
Ice-cold propanol-2 (100 mL) was added to 100 mL of culture supernatant fluid in a 250 mL centrifuge tube, kept on ice for 30 min, and centrifuged at 10,000 rpm for 15 min. The pellet was collected and washed with 50% (v/v) of propanol-2 in electrophoresis buffer and centrifuged again at 10,000 rpm for 15min.The pellet was collected and dissolved in 10 mL of electrophoresis buffer (EB; 10 mM Tris-HCl, 1mM EDTA, both Sigma-Aldrich, UK, pH 8). The sample was placed in a dialysis tube (Medical International Ltd, UK) and dialysed against EB for at least 2 h at 5 ºC. The preparations were removed and stored at 0 ºC until used for electrophoresis.
Electrophoresis
Sample (250 μL) was mixed with 250 μL of 5 % (w/v) SDS and 10 % (v/v) β-mercaptoethanol in electrophoresis buffer solution (Pharmacia Biotech, UK) in a 1.5 mL Eppendorf tube and boiled for 5 min. SDS-PAGE (Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis) was performed using Phastgel 10-15 gradient polyacrylamide gels (Pharmacia) with a continuous 10-15 % gradient and 2 % cross-linking. Protein preparations (3 µL) were applied and electrophoresis performed at 250V (10.0 mA) for 60 Vh. Protein markers (α-lactalbumin 14,000; trypsin inhibitor, 20,000; carbonic anhydrase, 30,000; ovalbumin, 43,000; bovine serum albumin, 67,000; phosphorylase b, 94,000: Amersham Biosciences, UK) were included on each gel for calibration.
Detection of proteins
The proteins were detected by staining with silver stain (Amersham Biosciences, UK). All staining solutions were prepared freshly before use as direction provided with the kit. The gels were washed x 3 with distilled water for two min on a rotary shaker. Dry gel solution (35 mL; Invitrogen Ltd) was added and shaken for 15 min. The gel was placed between two cellophane sheets (Invitrogen Ltd) and left overnight to dry.
The gels were photographed using an Alpha Imager™2200 system (Alpha Innotech Corporation, UK) and the Rƒ values and the molecular weight of each protein band was automatically calculated using the Alpha Imager™2200 and Alpha Ease™ software using the protein markers to calibrate each gel.
Estimation of protein concentration
Protein concentration was measured by the Bradford method (14) using Bradford Reagent and Bovine serum albumin as standard (both from Sigma Aldrich, UK).
Results
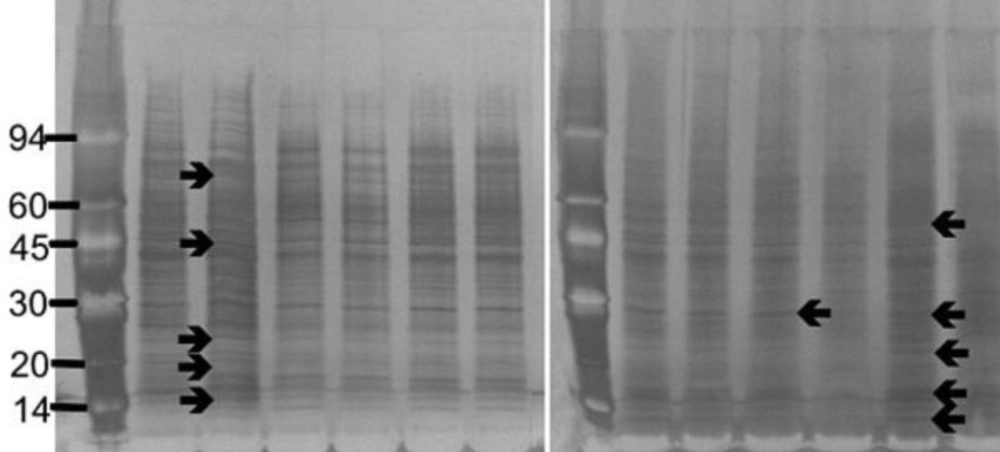
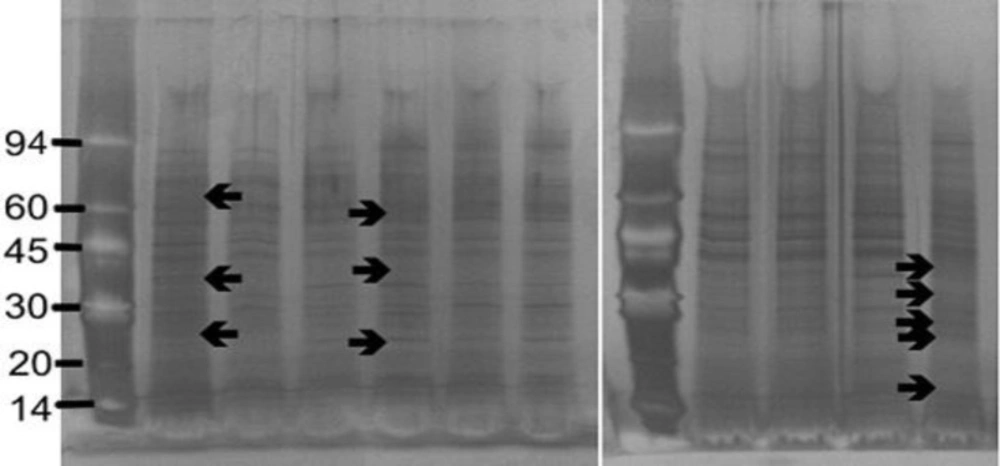
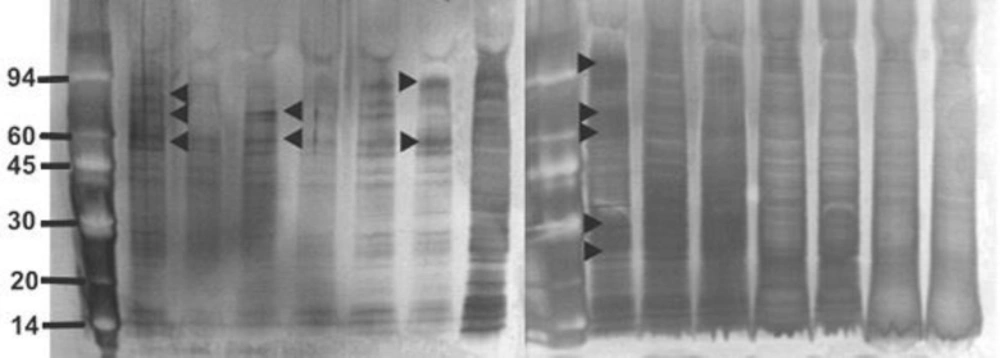
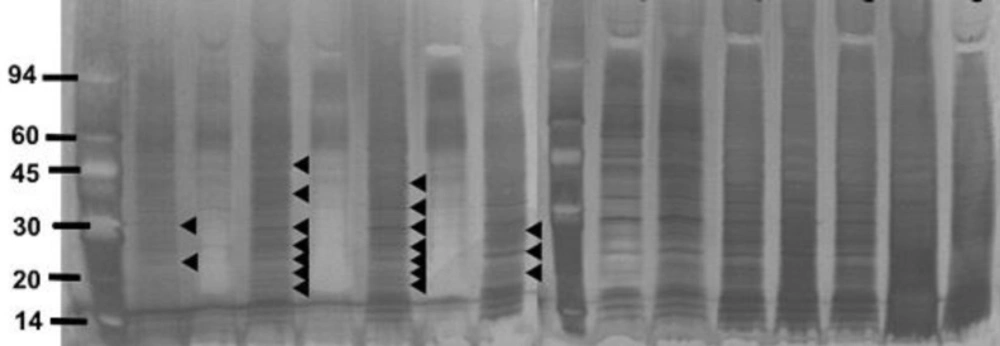
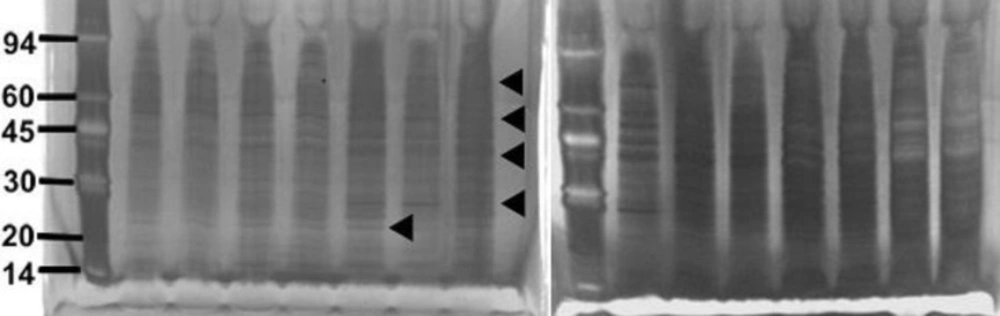
The strains of S. aureus were cultured for 24 h with and without a sub-inhibitory concentration of AgSD and exoproteins isolated from the culture supernatants. The total amounts of exoprotein varied from 8.7 to 13.3 mg 100 mL-l. Total protein remained the same with AgSD in the non-responder strains but increased by 6-15% with AgSD in responder strains. In strain B10, which does not produce TSST-1 and cannot be classified as a responder/non-responder, total exoprotein increased by 5 % in the presence of AgSD which proteins were fractionated by SDS PAGE. There was no clearly detectable increase in overall protein production in responder strains compared to non-responder strains. There were three patterns of exoproteins produced when cultures were grown with and without AgSD. All three strains previously identified as non-responders showed few detectable changes in the proteins (T1, B3, B35; Figure 1). Three strains previously identified as responder strains (T4, B54 and B23), showed a visible increase in the relative amounts of some proteins (arrowed). Some responder strains showed a reduction with AgSD (RN4282, B54) and two responder strains showed no change (B6, B45). Strain 8325-4 had not previously been classified and B10 did not produce TSST-1, both showed a reduction with AgSD (Figure 1). Most strains produced 20+ detectable bands but some bands were unique to particular strains.
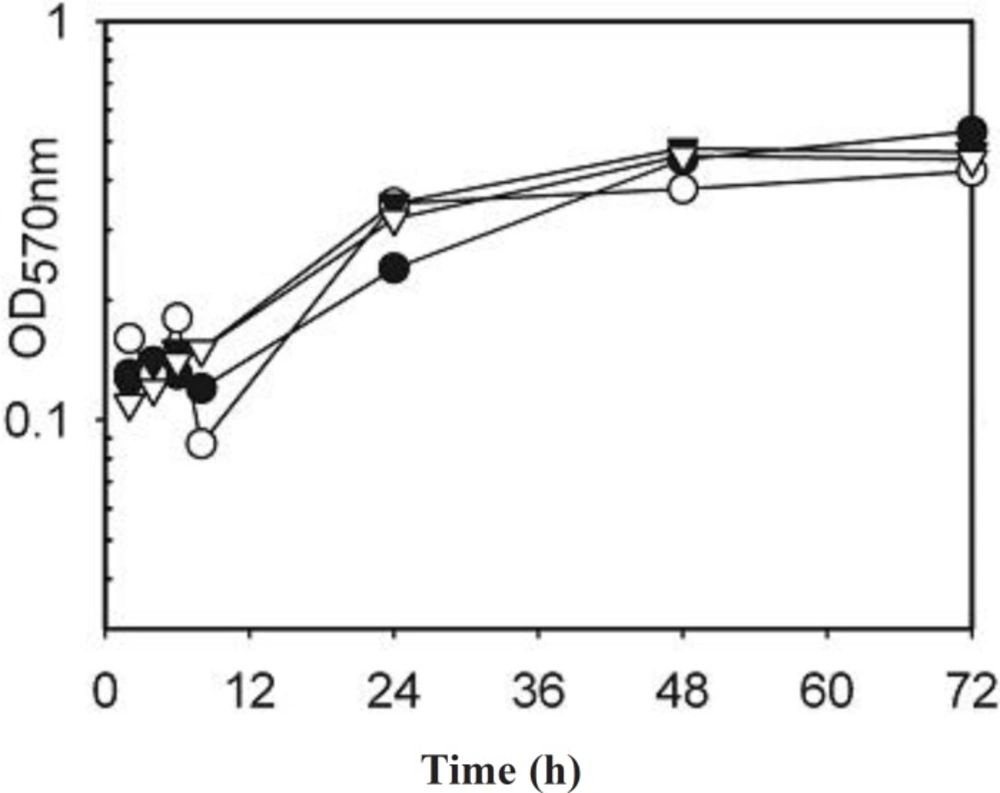
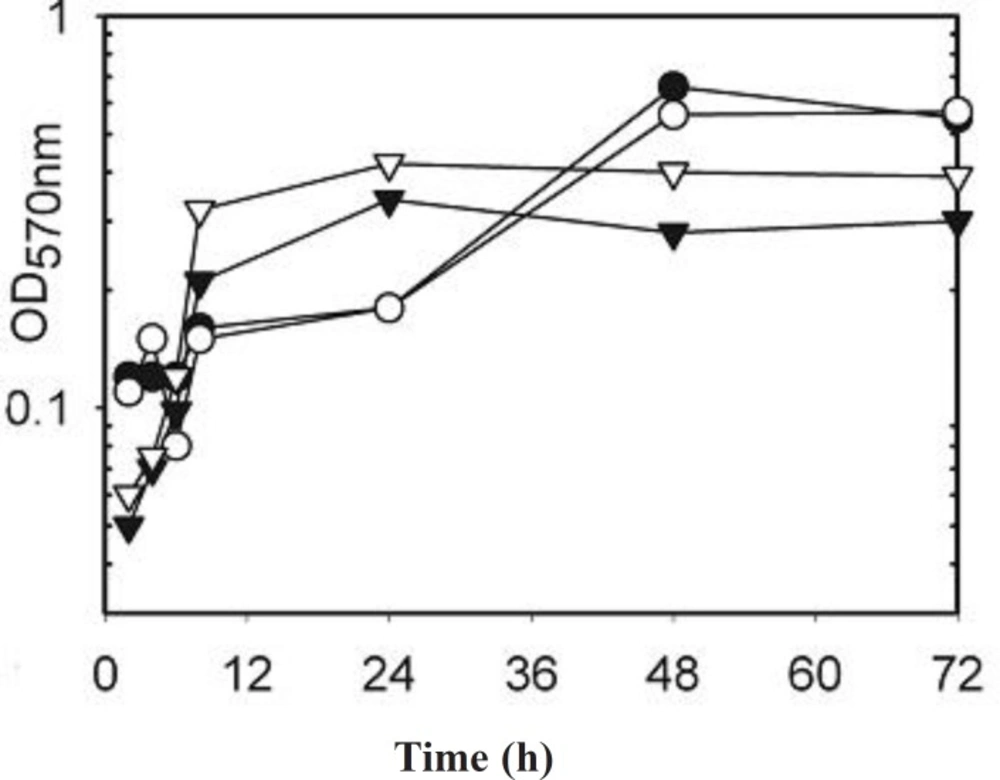
As the differences may have been due to changes in growth rate between the strains and between different strains the electrophoresis was repeated for strains incubated with and without AgSD for 2 to 72 h and the growth curves determined (Figure 2). There was generally little effect of AgSD on growth but there were some differences in growth rate between strains (Figure 2). Strains B10, T1 and B45 had similar growth curves and had entered stationary phase by 48h, strain B45 was the only strain to show a decline after 48 h. Strains RN4282, B3, B23, B54 and 8325-4 had similar growth curves, grew more gently and were still in exponential phase by 72 h. Strains B6, B35 and T4 showed growth decelerating after 24h and were in stationary phase by 48 h (Figure 2). The kinetics of exoprotein production in three representative strains is shown in Figures 3. In the responder strains e.g. T4, there was inhibition of exoprotein synthesis by AgSD up to 4 h, especially of proteins of 60-90 kD (Figure 3). Stimulation of proteins of 60 and 94 kD occurred after 6h and there was a general stimulation of protein by AgSD after 8 h, especially proteins of 22, 30, 60, 70 and 97 kD. There were few differences with and without AgSD at later times (Figure 3a). In contrast, in the responder strain B6, production of exoproteins was reduced in the presence of AgSD up to 8 h, particularly in the range of 15-45 kD. Amounts had recovered by 24h and there were few further differences up to 72 h (Figure 3b). In non-responder strain T1, there were few differences during the growth cycle except for reduced amounts of a band at 22 kD at 6h and bands at 40, 50 and 70 kD after 8 h (Figure 3c).
It was possible to tentatively identify some of the proteins on the basis of their molecular weight by comparison with published data (12, 13).
Figure 1. SDS PAGE of extracellular protease from Staphylococcus aureus strains culture incubated with and without silver sulphadiazine for 24h.
Figure 2. Growth curves of Staphylococcus aureus strains incubated with and without AgSD for 2 to 72 hours.
Figure 3. SDS-PAGE of extracellular proteins produced by Staphylococcus aureus Strains at different times 2, 4, 6, 8, 24, 48, 72 hours of incubation in the presence and absence of silver sulphadiazine (25 μg mL-1AgSD).
Discussion
The effects of sub-inhibitory concentrations of silver sulphadiazine on exoprotein synthesis were complex. There were three classes of response, responder strains that showed enhancement of synthesis of certain proteins, non-responder strains that showed no change and responder strains that showed a general decrease in exoprotein production in the early stages of growth.
The extracellular proteins produced by S. aureus strains were dissimilar in different growth phases. Some of the exoproteins were produced throughout growth, some were only produced in exponential phase, some were produced only in late exponential phase and some were only produced in stationary phase in both responder and non-responder strains. A number of factors have been shown to affect exoprotein synthesis. Expression of hla, tst and spa was strongly repressed in the presence of sodium chloride (1 M) or sucrose (20 mM), expression of tst requires O2 and CO2 and is affected by concentration of Mg2+ (15-19).
The synthesis of many exoproteins is regulated by agr and sar, so that is highest in stationary phase which was reached by most stains at 48 h in the present study. The cell density was approximately 10 to 20 times lower than in shaker (aerated) cultures (unpublished observations) but cell densities are still higher than the1.2 x 108 cfu mL-1 needed for induction of agr by 24-48h depending on the strain.
The control of exoprotein gene expression is a complex interplay between different regulatory systems (20-28). Sar regulates expression of agr mainly during the transition between exponential and stationary phases of growth and also has direct effects on expression of some exoproteins independent of agr (29-32).
It has also been reported that strains that produce TSST-1 produce lower amounts of extracellular proteins (32-34). No mechanism has been proposed for this. In the present study the TSST-1 negative strain B10 did not produce significantly more exoprotein than the TSST-1 producing strains. The reduction in exoprotein production in TSST-1 positive strains may not be a general phenomenon or may not have been expressed under the growth conditions used here.
The increases seen in this study were modest compared to the 8-32 fold increases in TSST-1 and changes in proteases seen in our previous study (12, 13). One difference is that static cultures were used in present study, whereas shaken cultures (aeration) were used in previous studies. The production of TSST-1 has been shown to be affected by aeration and CO2 levels (18, 19)and specific growth rate (33) and there is clearly an interaction with the stimulation of TSST-1 synthesis by AgSD and aeration which is not simply due to the higher cell densities formed by aerated cultures.
Overall the effects of sub-inhibitory concentrations of AgSD were complex with three classes of responses recorded in the strains tested. This may reflect differences in susceptibility to AgSD and, although MIC’s were similar, this could be tested by investigating the effects of different concentrations of AgSD. The results support the work of Kaur et al. (34) who showed that 70 μg/mL of Ag+ decreased synthesis of haemolysin, deoxyribonuclease and protein A but increased coagulase production in that the effect of Ag+ are dependent on exoprotein.
Some of the proteins were tentatively identified on the basis of their apparent molecular weight when compared to published data but there were a number of proteins that could not be identified. The pattern of exoproteins will depend on whether a particular strain carries the gene for a particular protein and may be complicated by activation of exoenzymes by cleavage by proteases e.g. lipase and serine protease (35) and by modification of surface proteins such as FnbA and protein A by proteoytic cleavage (27) the cleavage products of which will be present in the medium. The proteins that could not be identified from results published in previous studies may reflect the use of different strains and culture followed by MALDI/TOF (Matrix Assisted Laser Desorption Ionization/ Time of Flight) mass spectroscopy should allow identification of the proteins.