Introduction
Infection is a common complication in critically ill patients following hospital admission especially in intensive care unit (ICU). Antibiotics are widely prescribed for control of the patients’ infections in this ward. In addition to antibiotic resistant issue, adverse drug reactions are important concern regarding these drugs. Most adverse effects of antibiotics are mild and reversible but serious onessuch as acute kidney injury, acid base disturbances and electrolyte abnormalities can occur in these patients. Acute kidney injuryhas been reported with many antibiotics, particularly aminoglycosides (AG) and vancomycin. Several mechanisms are proposed for drug-induced acute kidney injury including acute tubular necrosis, allergic acute interstitial nephritis andvasculitis. Prevention of drug induced kidney injury and consequent electrolytes imbalances can decrease patients’ hospitalization costs (1-3). Aminoglycosidesare still widely used for treatment of gram-negative sepsis as combination therapy due to their synergistic effects (4). Acute kidney injury is common adverse effect of these drugs (5). Several strategies such asonce-daily dosing regimen, correction of volume depletion, hypomagnesaemia or hypokalemia before administration of these drugs and use of nephroprotective agentsare proposedto decrease AG-induced nephrotoxicity (6-8). The most reported electrolyte abnormalities related to AGare hypokalemia, hypomagnesemia, and hypocalcemia that are attributed to their renal tubular injury (9). Thecationic particles of AGattach to anionic membrane phospholipids, therefore lysosome swallow with phospholipid material and reduced generating of energy (10). It has been shown that atorvastatin may protect renal tubular cells from free radicals damage induced by gentamaicin (4). Intracellular isoprenoid pyrophosphates modified post-translation function of GTP-binding protein receptors. Isoprenoid pyrophosphates are metabolites of mevalonate that are made from the processing of mevalonate by 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase. Multi-ligand receptor megalin is a GTP-binding protein receptor that mediates endocytosis of AG. Atorvastatin inhibits HMG-CoA reductaseand maychanges intracellular isoprenoid pyrophosphates. Atorvastatin reduced AG- renal proximal tubule accumulation and cytotoxicity (11). In this study potential benefit of atorvastatin in prevention of amikacin-induced electrolytes imbalances has been evaluated.
Methods
This double-blinded, randomized clinical trial was conducted incritically ill patients hospitalized in general ICU of Imam Khomeini Hospital affiliated to Tehran University of Medical Sciences, Tehran, Iran, from June 2013 until June 2014. Adult patients (aged between 16-65 years old) who were candidate for AG (Amikacin) therapy for at-least 7 days were recruited. Included patients or their caregivers signed the study consent form and the Medical Ethics Committee of the hospital approved the study. Provided data are part of a RCT results registered in IRCT as IRCT201301283449N11.
Patients with renal impairment (eGFR<60ml/min), liver function dysfunction (liver enzyme serum levels over 5 times of the upper limit of normal), history of atorvastatin hypersensitivity reactions, positive history of drug induced myopathy or creatine phosphokinase over 5 times of the upper limit of normal, who received other nephrotoxic drugs or potential nephroprotective agent such as silymarin and vitamin E were excluded from thestudy.
Recruited patients were assigned to the atorvastatin or placebo group based on the simple randomization method. Atorvastatin group received amikacinwith dose of 15 mg/kg/day in two equal divided doses every 12 h as intravenous infusion during 30 min and atorvastatin 40 mg tablet as daily oral dose for 7 days. Patients in the placebo group received same dose of amikacinandplacebo tablet (Placebo group) for at least 7 days.
Demographic data (including age, sex, baseline diseases and causes of hospital admission) were extracted from the medical chartsand clinical characteristics (such as fever, hemodynamic parameters and type of infections anddrug regimens)of the included patients were monitored daily. Serum electrolytes (sodium, potassium, calcium, phosphor and magnesium) concentrations, blood urea nitrogen and serum creatinine levels were measured at day 0 and end of the study.
Considerable adverse drug reactions of atorvastatin including myopathy and hepatotoxicity were followed by measuring the patients’ serumcreatine phosphokinase and liver enzyme tests at baseline and end of the study. Increases in serum creatinine (doubling from the baseline value) were considered AG-induced acute kidney injury.
Data were analyzed using SPSS software version 14. Continuous data were expressed as mean ± standard deviation. Categorical variables were reported as percentages. Chi square or Fisher exact test (if more than 20 % of the categories have expected frequencies less than 5) was used for comparing categorical variables between the groups. Changes in the patients’ serum electrolytes (sodium, calcium, potassium, magnesium and phosphor) concentrations, blood urea nitrogen and serum creatinine before and after the interventionwere compared using paired sample t-test. Independent sample t-test was used to compare changes in the serum electrolytes between two groups of the study.
| Variables | Atorvastatin group | Placebo group | P value |
|---|---|---|---|
| Age ,mean ± SD | 59 ± 19 | 49 ± 18 | 0.074 |
| Gender (n) | |||
Male Female | 12 | 14 | 0.632 |
| APACHE II, mean ± SD | 20.3 ± 5.3 | 19.7 ± 4.4 | 0.684 |
| Mortality (n) | 5 | 4 | 0.401 |
| Chronic disease (n) | |||
Respiratory Diseases Cancer Cardiovascular diseases Neurological diseases Diabetes Hypothyroidism Total | 2 | 2 | 0.083 |
| Admission Diagnoses (n) | |||
Cancer Respiratory failure Cardiac problems Neurological disorders Trauma Abdominal complications Orthopedic surgeries’ complications | 8 | 8 | 0.49 |
| Culture results of the patients’ biological samples(n) | |||
Negative Respiratory Blood Urine Wound | 8 | 17 | 0.086 |
| Microorganism (n) | |||
Acinetobacter Enterobacter Pseudomonas Klebsiella Streptococcus viridians | 2 | 4 | 0.6 |
| Hemoglobin concentration (g/dl) | |||
Before treatment After treatment | 9.77 | 9.72 | 0.924 |
| Platelet count (cells/mm3) | |||
Before treatment After treatment | 239 ± 134 | 251 ± 159 | 0.792 |
| INR | |||
Before treatment After treatment | 1.25 ± 0.47 | 1.36 ± 0.53 | 0.473 |
| pH | |||
Before treatment After treatment | 7.42 ± 0.06 | 7.4 ± 0.08 | 0.185 |
| HCO3 (meq/l) | |||
Before treatment After treatment | 24.8 ± 3.5 | 24.9 ± 6.3 | 0.944 |
| Pco2 (mmHg) | |||
Before treatment After treatment | 40.4 ± 6.05 | 40.8 ± 10.6 | 0.89 |
| Sodium intake (mEq/day) | 413.4 ± 103.3 | 400.4 ± 117.6 | 0.705 |
| Potassium intake (mEq/day) | 24.2 ± 18.75 | 21.7 ± 19.7 | 0.674 |
| Calcium intake (mEq/day) | 15.79 ± 40.4 | 24.4 ± 39.8 | 0.484 |
| Phosphor intake (mmol/day) | 1.16 ± 2.77 | 1.84 ± 5.56 | 0.627 |
| Magnesium intake (mEq/day) | 5.3 ± 13.37 | 14.88 ± 22.6 | 0.088 |
| Antibiotic Regimens | |||
Vancomycin + Carbapenem + Amikacin Vancomycin + Tazocin + Amikacin Vancomycin + Cephalosporins + Amikacin Vancomycin + Amikacin Carbapenem + Amikacin Tazocin + Amikacin Carbapenem + Metronidazole + Amikacin | 10 | 15 | 0.467 |
| Indications ofamikacin administration Respiratory Sepsis Peritonitis | 17 | 16 | 0.143 |
| Other Drugs PPI Ranitidine Diuretics Hydrocortisone Vancomycin Carbapenems Tazocin Cephalosporins Inotropic agents | 14 | 21 | 0.401 |
| Metabolic support Parenteral nutrition Enteral nutrition Oral intake Parenteral and enteral nutrition | 2 | 6 | 0.663 |
| Fluid balance Input output | 3.3 ± 0.59 | 3.4 ± 0.52 | 0.686 |
| Liver function tests AST (IU/L) ALT (IU/L) | 40.79 ± 20 | 55.6 ± 40.4 | 0.152 |
| Muscle injury assessment CPK (mcg/L) | 33.18 ± 9.47 | 28.95 ± 16.87 | 0.371 |
Results
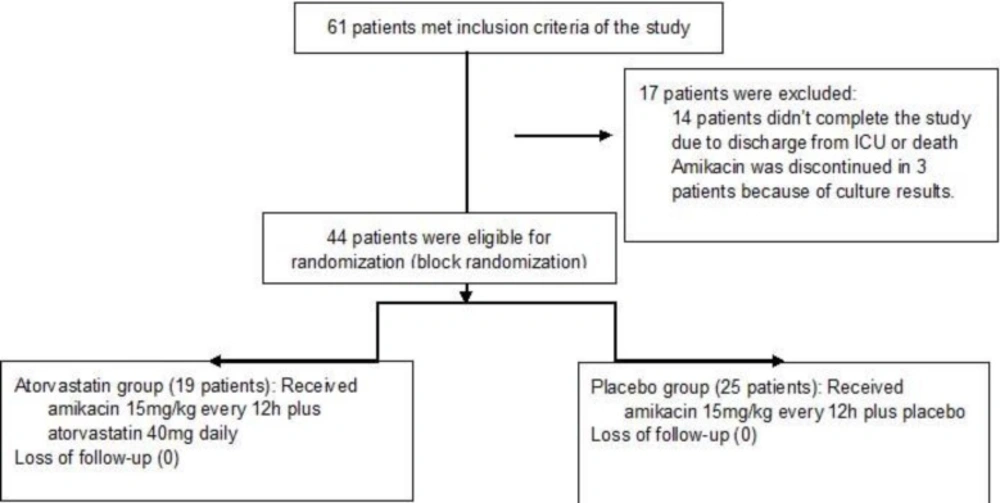
In the initial screening of the hospitalized patients in the general ICU, during one-year period, 61 patients met inclusion criteria of the study. During the study period, seventeen patients were excluded due to discharge from the ward (10 patients), death (4 patients) and discontinuation of amikacin (3 patients). Finally 44 patients (19 patients in the atorvastatin group and 25 patients in the placebo group) completed the study (Figure 1). The patientsmean±SDof age in theatorvastatinand placebo groups was 59 ± 18 and 49 ± 19 years respectively (p=0.07).There was no significant difference in the patients sex between the groups (p=0.63). The patients’ severity of the diseases based on the APACHE score II (acute physiologic and chronic health evaluation) was not different at the admission time (p=0.68). The patients’ types of the baseline diseases and diagnosis at the admission time were comparable. Cultures of the biological samples (blood, urine, respiratory tract secretions or wound) were positive in 11 (57.89 %) and 8 (32.00 %) of the patients in the atorvastatin and placebo group respectively. Acinetobacter spp. (31.57 %), Klebsiella spp. (31.57 %), Pesudomanasaeruginosa(21.05 %) and Enterobacter spp.(10.52%) were the most common isolated microorganisms. Respiratory tract infections (mostly ventilator associated pneumonia (75.00 %), sepsis (22.72 %) and peritonitis (2.28 %) were indications for amikacin administration in our patients.
There was not any significant difference between the groups regarding the patients’ common laboratory data including hematologic parameters. Descriptive characteristics and laboratory parameters of the patients are summarized in the table 1.
Regarding the patients’ renal function during the study period, there was no significant change in the blood urea nitrogen (BUN) and serum creatinine before and after the intervention in the both groups.
At baseline, before initiation of the intervention, mean±SD of the patients' serum sodium concentration was 138 ± 5.52 and 136 ± 4.29 meq/lin the atrovastatin and placebo group respectively (p=0.95).In the both groups, serum sodium did not change before and after treatment significantly (P>0.05). Also difference between serum sodium concentration at the end of the study remained insignificant between thegroups (P = 0.56).
Baseline mean ± SDof serum potassiumconcentration in the atorvastatin and placebo group was 4.07 ± 0.37and 4.15 ± 0.53 meq/L respectively (p = 0.88). Serum potassium concentration remained unchanged at the end of the study in the atorvastatin group (P = 0.61) but significantly decreased from 4.15 ± 0.53 to 3.80 ± 0.55 meq/L in the placebo group at day 7(P = 0.02). However difference of the serum potassium concentration at the end of the study was insignificant between the groups.
The patients’ serum calcium, magnesium and phosphor concentrationsdid not different between the groups at the baseline and day 7 of the study. Also changes in the concentrations of these electrolytes were not significant within each group during the study period. The serum electrolytes concentrations of the included patients are summarized in the table 2.
| Electrolyte | Atorvastatin group | Placebo group | ||
|---|---|---|---|---|
| At baseline | After treatment | At baseline | After treatment | |
| Serum sodium concentration (mEq/L) | 138 ± 5.52 | 135.8 ± 4.60 | 136 ± 4.29 | 136.6 ± 6.50 |
| P value | 0.096 | 0.719 | ||
| Serum potassium concentration (mEq/L) | 4.07 ± 0.37 | 4.17 ± 0.63 | 4.15 ± 0.53 | 3.80 ± 0.55 |
| P value | 0.61 | 0.02 | ||
| Serum calcium concentration (mg/dL) | 7.5 ± 1.01 | 7.66 ± 0.88 | 7.4 ± 0.48 | 7.8 ± 0.82 |
| P value | 0.352 | 0.23 | ||
| Serum phosphor concentration (mg/dL) | 2.94 ± 0.39 | 3.36 ± 0.59 | 2.96 ± 0.52 | 3.45 ± 0.4 |
| P value | 0.074 | 0.021 | ||
| Serum magnesium concentration (mg/dL) | 2.2 ± 0.26 | 2.3 ± 0.44 | 1.86 ± 0.31 | 2.06 ± 0.29 |
| P value | 0.423 | 0.094 | ||
| Serum creatinine concentration (mg/dL) | 0.76 ± 0.2 | 0.76 ± 0.2 | 0.76 ± 0.3 | 0.76 ± 0.3 |
| P value | 1 | 0.94 | ||
| BUN concentration (mg/dL) | 36.1 ± 15.4 | 40.1 ± 26.5 | 36.4 ± 20.5 | 39.2 ± 23.5 |
| P value | 0.494 | 0.57 | ||
Discussion
Intra-and extracellular concentrationsof electrolytes are necessary for many metabolic processes and maintenance of the normal organ functions (12). Electrolytesimbalances are common in critically ill patients. Several complications such as respiratory failure, edema, muscle weakness, altered mental status, and arrhythmiashave been reported following electrolytes imbalances in the patients hospitalized in ICU (12).
Normal function of mostvital organs especially kidney are necessary for the human body lectrolytesregulation (13). In addition to the body organs dysfunction, electrolytesimbalances could be consequence of administration of some medications such as AG antibiotics. Electrolytes imbalancesare associated to renal toxicity of AG (14-15). These drugsaccumulate in the epithelial cells in the renal cortex mainly in the proximal and distal tubulesand collecting ducts. Aminoglycosides enterto the mentionedcell by endocytosis through transporter of proteins and cations called megalin and cubilin (15-16). After influx, they accumulate mostly in lysosomes, the Golgi, and endoplasmic reticulum. When the concentration of AG in the endosomalsystems exceed, their membrane is disrupted and their contentis released into the cytosol. Cytosolic AGact on mitochondria and stimulate the intrinsic pathway of apoptosis, interfere withcell’ respiratory chainand reduce ATP production, which further cause cell death (18). Electrolytesim balances associated with AGconsisted of hypokalemia, hypomagnesemia, and hypocalcemia. The exact mechanisms of AG-induced electrolytesabnormalities are unknown. It seems renal tubular chloride channel may stimulate by AG resulting in excessive urinary chloride loss. Following sodium chloride wasting, renin-angiotensin- axis is stimulated and subsequently hypokalemic metabolic alkalosis occurs. Hypokalemia may induce hypomagnesemia (1). In addition, AG candecrease brush border membrane enzymes and phospholipids and consequentlydecrease transport of organic bases, electrolytes (sodium, potassium and calcium) and also decrease of Na-KATPase activity (15).
Intracellular isoprenoid pyrophosphates modified post-translation function of GTP-binding protein receptors. Isoprenoid pyrophosphates are metabolites of mevalonate that are made from the processing of mevalonate by 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase. Multi-ligand receptor megalin is a GTP-binding protein receptor that mediates endocytosis of AG. Atorvastatin inhibits HMG-CoA reductaseand consequentlychanges intracellular isoprenoid pyrophosphates. Based on this mechanism, atorvastatin results in reduction in AGrenal proximal tubule accumulation and cytotoxicity (11). In 2009, Emin Ozbek et al. study results showed that atorvastatin mayprotect kidney of rats from free radicals induced by gentamaicin (4).
Renal toxicity was not detected in any patient in the both groups. Serum potassium concentration remained unchanged after treatment in the atorvastatin group but was decreased in the placebo group following 7 days of amikacin therapy. Hypokalemia is reported as the most common type of electrolyte imbalances following AG therapy (17-20).
Small sample size and short duration of the patients’ follow-up are the major limitation of this study. Also we did not measure urine electrolytes and effects of AG and atorvastatin on the urinary electrolytes loss.
Present study is first human randomized clinical trial that has evaluated possible effects of atorvastatin in prevention of AG-induced electrolytes imbalances. During a 7-day course of amikacin therapy we did not detect any renal adverse effects including acute kidney injury or electrolytes imbalances except decreasing serum potassium concentration. In this pilot study, atorvastatin as 40 mg daily oral dose prevented renal potassium loss during course of amikacin therapy in the critically ill patients. In the future well designed randomized clinical trials with adequate sample size and longer duration of patients’ follow-up, renoprotective effects of statins should be examined.