Introduction
Venous thromboembolism (VTE) includes deep vein thrombosis (DVT) and pulmonary embolism (PE). It is common among patients with cancer, immobilization and major surgery (1). VTE is a common cause of mortality and morbidity in hospital but it is preventable (2). The incidence of venous thromboembolism exceeds 1 per 1000; over 200,000 new cases occur in the United States annually (3).
Intravenous heparin or low molecular weight heparin (LMWH) followed by at least 3 months oral anticoagulant therapy is standard treatment for acute VTE (4). Traditional anticoagulants, Vitamin K antagonist (VKAs) such as Warfarin are considered in this period for many years because they are effective in prevention and treatment of venous thromboembolism, as well as prevention of systemic embolism and stroke (5). Nevertheless, Warfarin has narrow therapeutic window, extensive drug and food interactions, slow onset and offset of action, lack of selectivity for coagulation factors and need monitoring frequently. The pharmacological response is also unpredictable and highly variable among patients base on genetic, ethnic etc (-). Also Warfarin caused 33% of emergency hospitalizations for adverse drug events in older patients (11).
New oral anticoagulants such as pharmacologic agents which directly inhibit factor II (thrombin) or Factor Xa have been studied for prevention of thromboembolic disorders. These drugs provide many benefits rather than vitamin K antagonists (VKAs) due to pharmacological differences, monitoring, food interaction, drugs interaction and etc (12, 13). Dabigatran as oral predictable anticoagulant drug, have been approved by food and drug association (FDA) for stroke prevention and systemic embolism in patients with non-valvular atrial fibrillation (14). But since now this drug haven’t approved for treatment of VTE by FDA. General objective of this study was systematic review comparing side effects of Dabigatran versus Warfarin in treatment of acute venous thrombosis.
The following were set as the specific objective of the study:
Comparing death during therapeutic period between two groups.
Comparing recurrent thrombosis during therapeutic period between two groups.
Comparing major bleeding during therapeutic period between two groups.
Comparing minor bleeding during therapeutic period between two groups.
Methods
Following criteria are considered for study:
Type of studies: randomized controlled trials (RCTs) were selected to compare Warfarin versus Dabigatran in treatment of venous thromboembolism.
Type of participant: patients with proven VTE.
Type of intervention: Dabigatran as oral direct thrombin inhibitor versus Warfarin.
Type of outcomes: mortality, recurrent embolism, major and minor bleeding.
Database Search for selection of RCTs:
We searched Ovide, PubMed, Cochrane (CENTRAL), EMBASE, Scopus, Science Direct LILAC (for article written not English) and also Iranian database Magiran, ISC, IranMedex, IranDOC, Doaj up to may 2014. We also we checked Request database for thesis. No language restrictions were considered. References of the related articles and complete reviewed articles, were also investigated. Two investigators evaluated trials separately and independently for eligibility and extracted data. The keyword for search strategy are available in appendix 1.
Study selection
We included randomized controlled trials (RCT) compared Dabigatran with standard treatment of acute VTE Warfarin (dose-adjusted to maintain an INR between 2.0-3.0) with 5 days overlapped of SC LMWH or IV heparin. Two authors separately evaluated the title and the abstract which were collected by the electronic researches.
Data extraction and quality assessment:
We collected outcome data according to the following subgroups;
Primary outcomes: related death, recurrent Thromboemboly
Secondary outcome: major bleeding events (intracranial, intramuscular…), minor bleeding events (intracranial, intraocular, urogenital…), acute coronary syndrome.
Also, we collected the data of patient characteristics form trial populations; age, race, body mass index, estimated creatinine clearance, cancer at base line and previous venous thromboembolism.
We assessed study quality of clinical trials using CONSORT (checklist for RCT) available in appendix 2.
Data synthesis and analysis
We considered direct comparisons between Dabigatran versus standard treatment (Warfarin) on an intention to treat basis, according to PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) recommendations(15). For meta-analysis results were similar to the second article because researchers have done pooled analysis of two studies.
Results
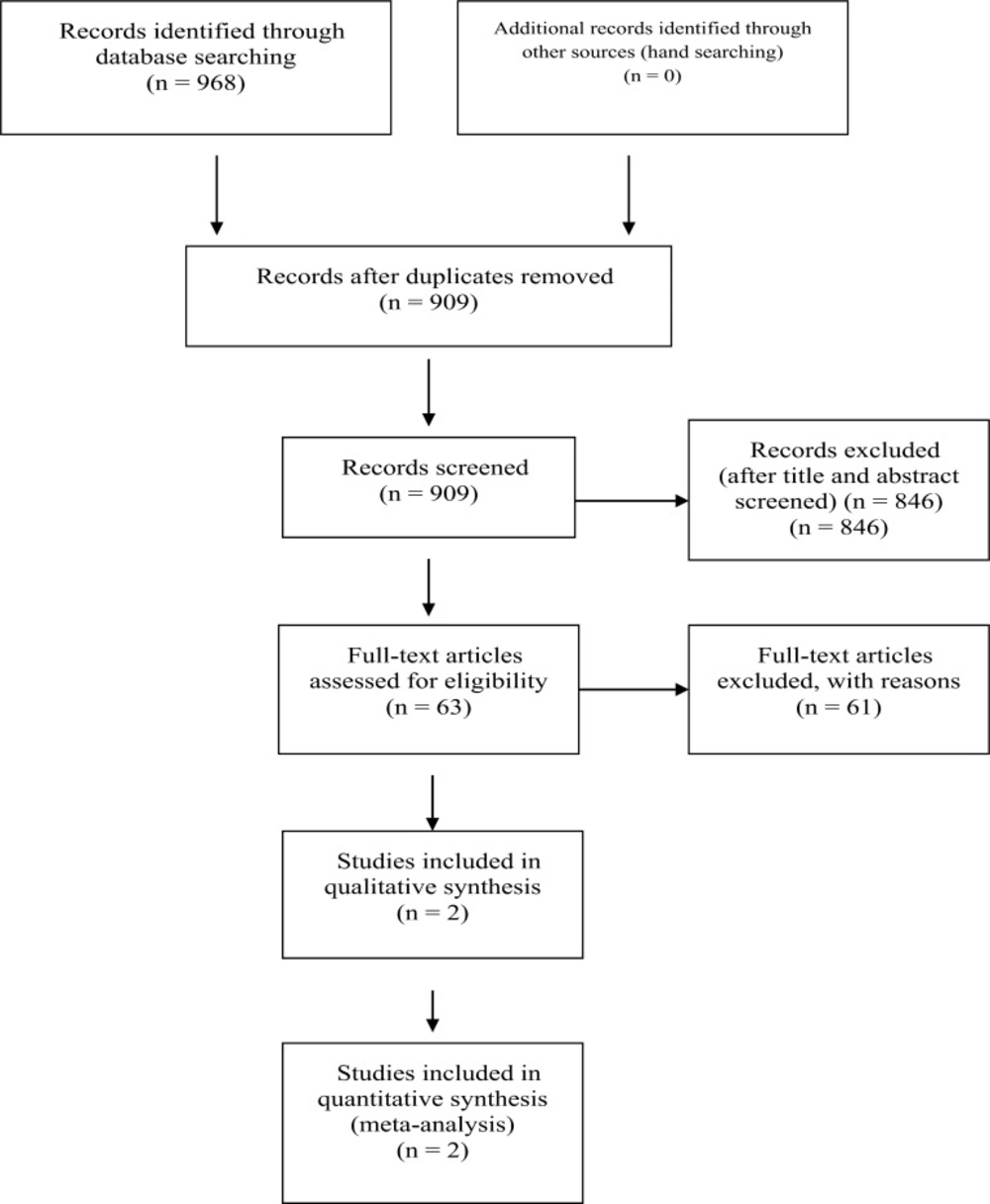
The systematic review identified 909 articles, sixty tree article were selected to read full text. Finally 3 articles were selected but two studies include in systematic review (RE-COVER and RE-COVER II) (16, 17) . One of the tree articles exclude because the population study was very small (55 patient against 5107 patient in both articles) and outcomes was different, although the study was RCT and researcher worked on Dabigatran and Warfarin (18).
Characteristics of trials, treatments and outcomes measures
The two studies comprised 5107 randomized patients and compared Dabigatran (n = 2,553) with standard treatment, Warfarin (n 2554) (16, 17). Table 1 shows the characteristics of the trials and treatments. Methods for diagnosing of recurrent VTE were done in studies (16, 17). The diagnosis of VTE was established by using of compression ultrasonography or venography of leg veins and ventilation-perfusion lung scanning, angiography, or spiral computed tomography of pulmonary arteries that were done before randomization. Recurrent venous thromboembolism were diagnosed with the use of the same diagnostic methods that had been used for the initial diagnosis. The team defined major bleeding according to the International Society on Thrombosis and Haemostasis criteria (19). Other bleeding was defined relevant non-major bleeding or as nuisance bleeding.
| Study name | No in samples | Patients | Experimental treatment | Control treatment | Duration of treatment | Design | Risk of bias |
|---|---|---|---|---|---|---|---|
| RE-COVER | 2564 | All acute VTE | Heparin 5 days and until (sham) INR is 2.0, followed by DAB 150 mg BID | Heparin 5 days and until | 6 months (mean: 5.6) | Double-blind | Low |
| RE-COVER II | 2589 | All acute VTE | Heparin 5 days and until (sham) INR is 2.0, followed by DAB 150 mg BID | Heparin 5 days and until | 6 months (mean: 5.6) | Double-blind | low |
Patients' characteristics and quality of anticoagulation
Mean patients' age in RECOVER and RECOVER II were 55 and 10% of patients were 75 years or older with a predominance of male gender 58 and 61 percent in RECOVER and RECOVER II respectively (Table 2). Active cancer was present 4.7% and 3.9% of patients at baseline. Moderate renal insufficiency was present in 5% of patients. Previous history of VTE was seen at 25.5 and 17.5 % of patients in RECOVER and RECOVER II respectively.
The International Normalized Ratio (INR) was within therapeutic range (2 to 3) percentage of time within therapeutic range (TTR) in RECOVER 60% and RECOVER II 57% (Table 2). TTR during the first month was 53% and 51% and end of study 66 and 62 in RECOVER and RECOVER II respectively (Table 2). Although over all the efficacy of Dabigatran and Warfarin was similar and statistically not difference at any age. Other Characteristics of patients like sex, ethnic, body mass index, creatinine clearance… not influence on treatment effect.
| Study | No. | Cancer at | Mean | Mean | Male gender (%) | Active cancer (%) | CrCl< 50 ml/min (%) | History of | TTR (%) | TTR (%) in first month | TTR (%) end of study |
|---|---|---|---|---|---|---|---|---|---|---|---|
| RE-COVER | 2564 | (121) 4.7% | 55 | 83 | 58 | 5 | 5 | 25.5 | 60 | 53 | 66 |
| RE-COVER II | 2589 | (100) 3.9% | 55 | NA | 61 | 4 | 5 | 17.55 | 57 | 51 | 62 |
Recurrent VTE events
For the 2 studies combined, Dabigatran at least as effective as Warfarin in preventing recurrent venous thromboembolism or related death, During 6 month (Hazard Ratio (95% CI)1.09 (0.76-1.57)).
Bleeding events
Significant relative risk reductions were seen by Dabigatran for clinically relevant non-major bleeding. Also Dabigatran reduced Significantly any bleeding versus Warfarin in patients, however gastrointestinal (96 vs 68) and Retroperitoneal (7 vs 2) bleeding by Dabigatran was higher than Warfarin.
Major bleeding was not significant different however the number of patients in Dabigatran group were less than Warfarin group (Table3).
| Outcome | Dabigatran (n/N) | Warfarin (n/N) | Hazard Ratio (95% CI)* |
|---|---|---|---|
| Major bleeding event, n subjects (%) | 37/2553 | 51/2554 | 0.73 (0.48-1.11) |
| Any bleeding event, n subjects (%) | 411/2553 | 567/2554 | 0.70 (0.61-0.79) |
The hazard ratio was estimated with the use of the Cox model with factor treatment stratified by study, assuming different baseline hazards per study.
Deaths and cardiovascular events
Death in both groups was similar and statistically not significant (Hazard Ratio (95% CI); 1.0 (0.67-1.51)). There were higher numbers acute coronary syndrome in Dabigatran group; 17 versus 9 however statistically not significant. This Scientific findings were seen prior in other trials (20, 21).
Discussion
VTE treatment includes initial injectable anticoagulants, followed by oral anticoagulation with Warfarin. Warfarin therapy is dosed and monitored according to therapeutic response as measured by the international normalized ratio (INR) (22). Monitoring for adverse effects including hemorrhage is also critical. But this therapy is influenced by multiple factors, and patients on Warfarin require ongoing education to maintain safe and effective anticoagulation (23, 24). Initiation of Warfarin dosing is complex because dosing requirements vary significantly among individuals. Daily doses as low as 0.5 mg and as high as 20 mg or more may be required in individual patients to reach a therapeutic INR however an average dosing requirement of 4 to 5 mg/day of Warfarin is necessary to maintain an INR of 2.0 to 3.0 in most patients.
A number of oral direct thrombin inhibitors are being investigated as alternative options to Warfarin for stroke prevention in atrial fibrillation, prevention and treatment of venous thromboembolism, acute coronary syndromes, and other indications. Dabigatran is a new oral direct thrombin inhibitor, approved for stroke prevention in patients with atrial fibrillation (25, 26) Unlike Warfarin, Dabigatran is given at a fixed dose because of predictable pharmacokinetic profile without the need for routine coagulation monitoring or dosing adjustments (27). In patients with atrial fibrillation, a dose of 150 mg twice daily is used if creatinine clearance (CrCI) is > 30 Ml/minute, and lowered to 75 mg twice daily for CrCl15 to 30 mL/minute. Dabigatran is not metabolized by cytochrome P-450 (CYP) enzymes, it is not susceptible to CYP-mediated drug interactions (28). Different pharmacodynamic, pharmacokinetic and mechanism of Dabigatran caused to assay safety and effectiveness in VTE in many clinical trials. In this systematic review, comprising more than 5000 patients enrolled in two randomised clinical trials. Dabigatran were as effective as and generally safe than standard therapy of acute VTE, Warfarin. The only benefit of the Dabigatran was seen in the reduction of the minor bleedings however major bleeding in Dabigatran group was lower but not statically significant. In elderly patient(> 75 years), moderate renal failure (creatinine clearance of 30 to 49 mL/ min)and previous bleeding didn’t show increase risk in bleeding with Dabigatran, previously other articles showed these (29, 30).
The effect of ethnic in some articles like these articles were insignificant (28, 31) but it seems more research is needed because, despite the large number of patients randomized in these study (n = 5107), 82% were from Europe or North America and 85% of the study population was white. Influence of genetic factors on the inter individual variability in response to Warfarin (and numerous drugs) has been established so it was be clear that genetic polymorphisms varies among different populations and ethnic groups (32, 33).
Also, only 100 patients received Dabigatran and a permeability glycoprotein inhibitor in these research (2%) and there was no apparent increase in bleeding in this subset but some articles suggest caution when clinicians used Dabigatran in combination with strong inhibitors or inducers of P-glycoprotein, such as amiodarone or rifampicin (34). Although analysis did not show any effect of aspirin but some articles have been reported of interaction that Leading to death. Number of concomitant use of these drugs are less to decide for safety because absence of a reversal agent for Dabigatran raises concern for uncontrollable bleeding and death (35).
The increase risk of gastrointestinal hemorrhage may have a hint on patients with related predisposing factors. In this systematic review there were some limitation: researcher not hint to important topics, like: pregnant patient, impact on quality of life, cost estimated in clinic. Finally investigation of patient who need life time or long time anticoagulation such as heterozygous or homozygote patient factor 5 Leiden not considered. Further research would help to clarify these issues.
Conclusion
The result of this systematic review showed noninferiority of Dabigatran versus Warfarin for the prevention of recurrent VTE but perhaps safer than Warfarin as standard treatment of acute VTE. superiority of Dabigatran was seen for clinically relevant bleeding and for any bleeding but no for major bleeding(how ever it was lower but not statically significant).