Introduction
Chronic wounds are still a serious problem in patients referred to dermatologists or plastic surgeons. Diabetes is a common cause of delayed or impaired wound healing. In total, 15% of people with diabetes suffer from lower extremity wounds. Diabetic ulcers of lower extremity are associated with high morbidity and mortality (1). Reducing edema and improving the cutaneous microcirculation are appropriate treatment of lower extremity ulceration although prevention is the best treatment of lower extremity ulceration. Vitamin A (retinol) consists of a group of natural or artificial chemical compounds with hormone-like activity (1) that exit only in tissues of animals, but several types of herbal pigments generally called carotenoids, are converted to vitamin A in animals body (2). Retinol normally can be hydrolyzed in the intestinal lumen and then absorbed into the blood stream as chylomicrons. In addition, it can be irreversibly oxidized to produce retinoic acid in the intestine. Retinol is transported into the general circulation via the portal vein, and rapidly metabolized in the liver and kidneys and excreted via bile and urine (3). Transformation of inactive vitamin A (retinol) to the active retinoic acid is controlled by a cellular set that causes a balance in proper function of cells and tissue (4). Retinoic acids (RA), the active metabolites of vitamin, in the many stages of differentiation and proliferation of epithelial cells is involved (5). Despite these effects, its increased rate acted as a potent teratogenic in the fetus and induces a wide spectrum of abnormalities that called fetal damage induced by RA (6). All-trans RA (Tretinoin) has effects 100 to 1000 times more than retinol (7). This matter is an orangish- yellow crystal. The group of tretinoin through oxidation turn to carboxyl group that normally there is in human blood in very low concentrations (14-4 nmol. L-1) (8). Rosa damascena mill is one of the most important species of family Rosaceous or Rosa ceae (9). Several chemical compounds are extracted from Rosa, which include: trpuneens, glycosides, flavonoids and anthocyanins (10, 11). This plant also contains a carboxylic acid (12). The most important use of rose in the traditional medicine is treatment of abdominal pain, the heart reinforcement (13), polymenorrhea and gastrointestinal disorders (14), reduction of inflammation and ulcers skin (15, 16). Thus due to the effects of Retinoic acids and Rosa damascene the present study was therefore aimed at assess the combined effects of retinoic acid and Rosa damascena mill on wound in diabetic rats.
Experimental
Plant
Rose plant was prepared from herb garden of Ahvaz Jundishapur University of Medical Sciences (AJUMS) in the late spring season of 2012. Then, it was identified and verified by a scientific board member. Moreover, to prevent any destruction of its chemical compositions, it was kept in 20 °C.
Preparations of extract
The extract was prepared by maceration method. 100 gr of powdered Rose plant was soaked in 500 mL of 70 % ethanol and stirred intermittently for 48 h. at room temperature. The material was filtered using sterile cotton wool and Whatman (No. 1) filter paper. The pooled filtrates obtained were dried in oven at 40 oC for 48 h. and the extracted powder was kept at 4 oC until used (17). Finally 2 gr of powder was mixed with of 0.1 % tretinoein lotion.
Experimental design
Seventy -two rats (300-310 g) were prepared from animal house center and was used in this study in accordance with the Ethics Committee of AJUMS. Animals were maintained under controlled conditions of temperature (21±2 oC) and a 12-h light– dark cycle. They were fed normal rat diet and water. Diabetes was induced by a single intraperitoneal injection STZ (60 mg. kg1) dissolved in citrate buffer (o.1M pH 4.5). Three days after STZ injection, fasting blood glucose levels were determined with a glucometer (Easy Gluco Blood Glucose Monitoring system, Infopia, Korea). Rats with blood glucose more than 250 mg. dl-1 were chosen as diabetic animals (18). Diabetic rats were randomly divided, into three groups (24 rats in each group): control (A), positive control (B), case (C). In all groups, after anesthesia of the animals, a wound with full thickness of skin was carried through punch which the cut surface has 6 -mm of diameter were performed on back of each rat the vicinity the spine. Treatment was started on the first day after wounding in the groups of A, B and C respectively with normal saline solution, Phenytoin and a combined of 0.1 % Tretinoein lotion and hydro-alcoholic extract of Rosa damascena mill , once a day for 15 days. Each one of groups A, B, and C were divided into three subgroups with 8 rats. Subgroups were studied on days 5, 10 and 15. At the end of every one of these days, after anesthesia, the largest diameter of the wound was measured with a ruler and photographs of the wounds were taken. For microscopic evaluations, wound location was removed in dimensions 2 x 2 x 2 cm and were processed routinely for light microscopy. Two sections were made from each wound and stained with hematoxylin-eosin.
Measuring the area of the wound
Each animal was placed in a standard situation, then it was drawn with the help of the transparent paper and wound area was measured with the help of a ruler. The following formula was used to calculate the percentage of recovery:
Calculation method of healing
The calculation of healing based on macroscopic parameters: In this way, the wound area was measured from first day until the end of period. Then, the above formula was used to calculate the percentage of healing.
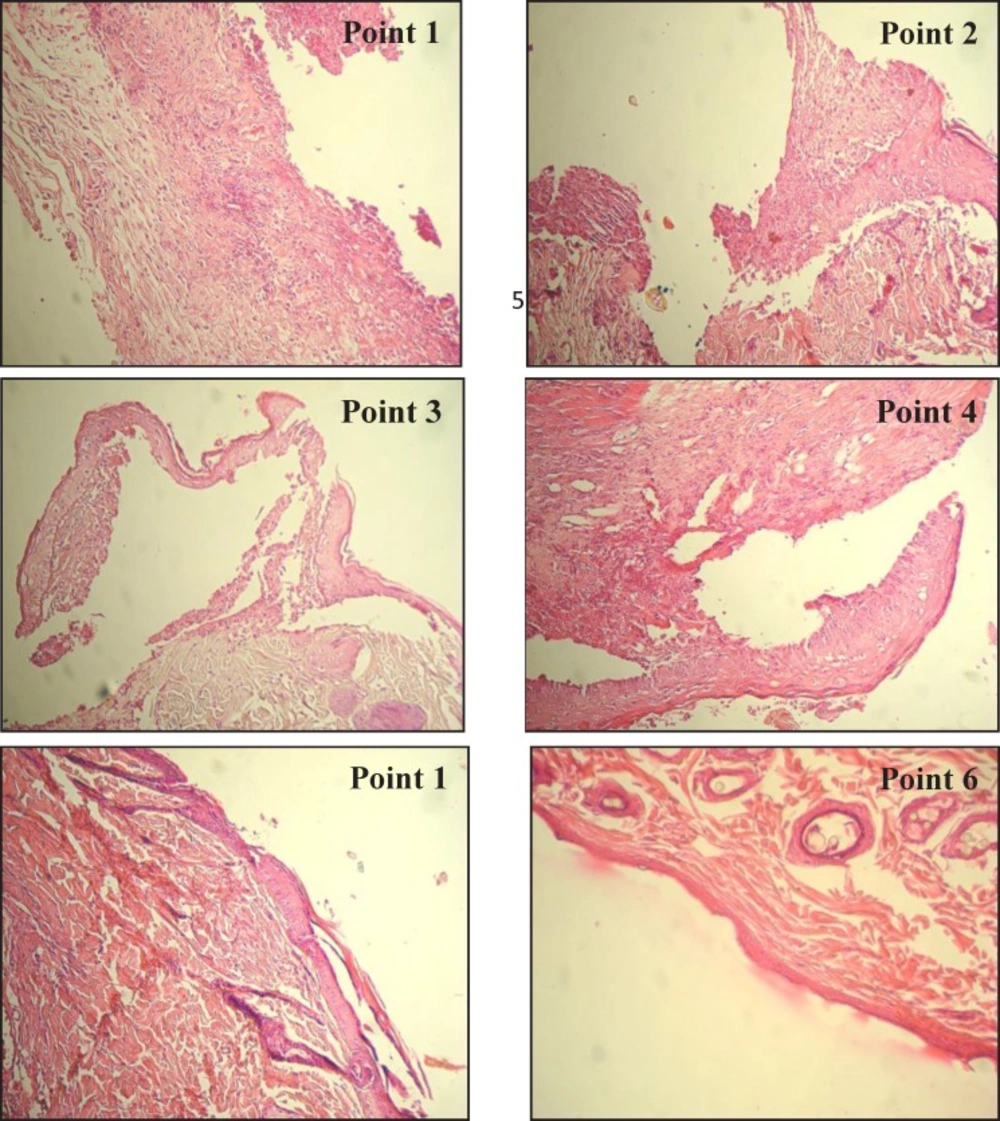
The calculation of healing based on microscopic parameter: the healing rate was determined by the pathological parameters that it was produced by the researchers according to various studies (Figure 1).
Point 1, there are not renewal of epithelial cells, formation of tissue bud and fibrotic tissue while an acute and mild inflammation may be seen in the wound.
Point 2, is associated with the start of epithelial cellsrenewal around and base of the wound, formation a little amount of fibrotic tissue around the wound and also acute and moderate inflammation of the wound may be seen.
Point 3, is associated with renewal of epithelial cells around of the wound more than the previous stage, formation a moderate amount of tissue bud and fibrotic tissue in the wound margin.
Point 4, is associated with the formation of bud tissue thoroughly and formation of fibrotic tissue more than the previous stage.
Point 5, is associated with renewal of epithelial cells completely, degradation of bud tissue and the formation of fibrotic tissue completely.
Point 6, at this stage the bud tissue is almost gone at this stage the bud tissue is almost gone, and only very mild inflammation may be seen.
Statistical Analysis
Data are expressed as the mean ± SE. Statistical significance of differences was assessed with one-way ANOVA by SPSS for Windows (version 17) followed by Tukey’s -test. P<0.05 was assumed as statistically significant.
Results
Macroscopic evaluation
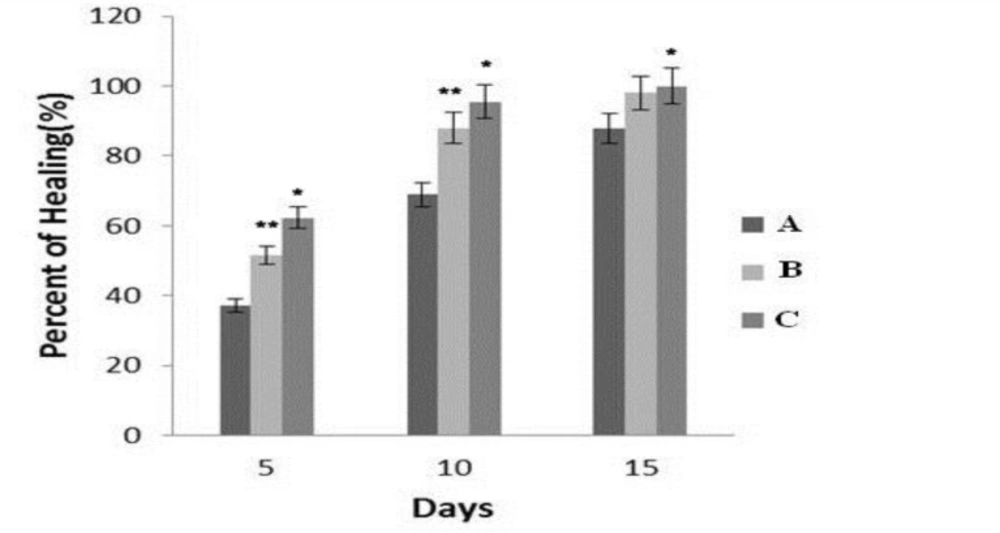
Macroscopic evaluation on the 5th day indicated that the wound size of the groups A, B, and C reduced to 4.33± 0.03 mm, 3.72 ± 0.02 mm, 3.03± 0.01mm respectively. So, the comparison of group C with groups A and B showed a significant reduction ((P < 0.003, P < 0.05) of in ulcer size in C group. In the evaluation conducted on 10th day, the wound size of the groups A, B and C reduced to 3.79 ± 0.02 mm, 1.52± 0.03 mm and 0.43 ±0. 01 mm respectively. Comparisons between groups A and C (P < 0.002) and also, between B and C (P < 0.04) showed a significant reduction in the size of wound in group C than the other two groups. In the study conducted on 15th day, the wound size of the groups A, B and C was decreased to 0.89 ± 0.1 mm, 0.82 ±0.01mm and 0.21±0.01 mm respectively. The comparison between the A and C showed a significant difference (P < 0.003), while did not find significant difference between groups B and C (P > 0.06). These results have been shown in Figure 2.
The comparison of mean ± SD in percentage of wound healing in evaluated groups on 5th, 10th and 15th days in different groups. A (normal saline solution), B (Phenytoin) and C (a combined of 0.1% Tretinoin lotion and hydro-alcoholic extract of Rosa damascena mill ). ٭compared to A group ( P < 0.05). ٭٭compared with C group (P < 0.05
Microscopic evaluation
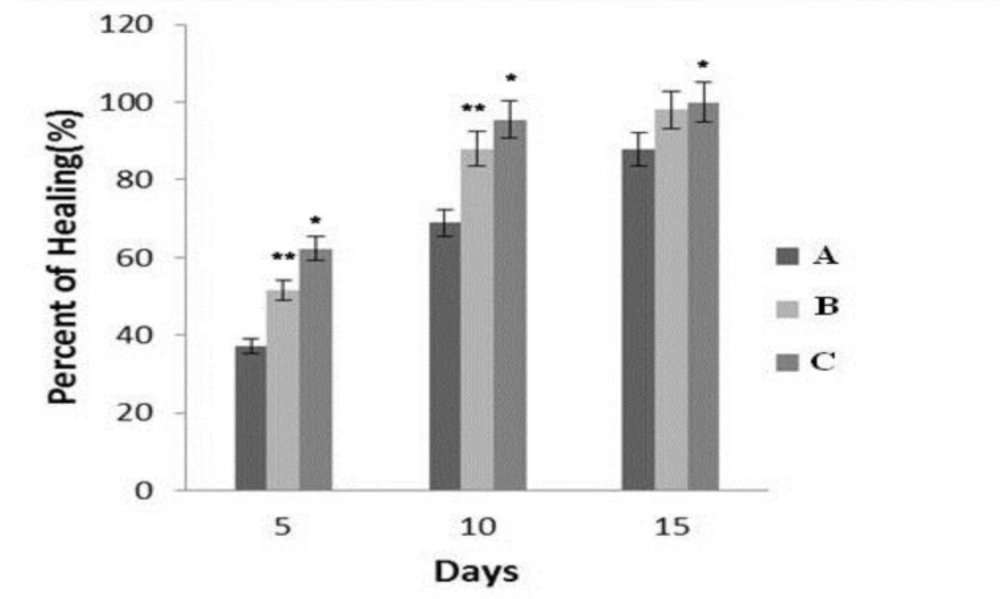
Comparison of groups on 5th day indicated a significant difference between groups A and C (P < 0.04) as well as B and C (P <0.05). In other word, the rate of wound healing in group C was higher than other groups. Comparison between groups on 10th day showed a significant difference between groups A and C (P < 0.04) like 5th day. while, the difference between groups B and C was not significant (P>0. 05). On the 15th day, a significant difference was found between groups A and C (P < 0.05) but, there was no significant differences between groups B and C (P > 0.05) (Figure 3).
The comparison of mean ± SD of scores given to pathological observations on the 5th, 10th and 15th days in different groups. A (normal saline solution), B (Phenytoin) and C (a combine of 0.1 % Tretinoin lotion and hydro-alcoholic extract of Rosa damascena mill ). ٭compared to A group ( p <0.05). ٭٭compared with C group (P < 0.05
Discussion
In the present study, the topical application of a combination of rose hydro-alcoholic extract and tretinoin accelerates wound healing markedly in diabetic rats. For more than 50 years, studies focused on the effects of vitamin A on wound healing (3). Tretinoin, a derivative of vitamin A, is commonly used in the treatment of acne vulgaris and skin aging. It also has significant effects on cellular differentiation, angiogenesis and collagen synthesis. Histologically, topical application of the tretinoin causes the hypertrophy of epidermis and derma through increasing of dermal vessels and influx of inflammatory cell (2, 19). Our findings showed that the topical application of a combination of 0.1 % tretinoin lotion and hydro-alcoholic extract of Rosa damascena mill for 5 days, dramatically enhanced wound healing after creating wound. Also, on the 10th and 15th days it was effective in wounds healing and reducing the size of wounds. We observed a decrease in size of wound in group C when compared to group B, but results of microscopic studies on the 10th and 15th days demonstrated unlike the 5th day, wound healing was not significant. It may be due to more effects of tretinoin in short term as discussed in a previous study (20). Previous study showed an increased amounts of collagen, vessels and tissue bud after treatment with the tretinoein (7). On the other hand, it has been shown that adding tretinoin improves some side effects of the treatment with steroids and radiation on the wound healing (21-24). In a study Kitano et al. demonstrated that topical application of retinoic acid has been associated with remarkable hypertrophy of epidermis and derma, angiogenesis and influx of inflammatory cell (3). Also other study indicated that wounds of the diabetic rats treated with trans-retinoic acid were closed faster than the group that were treated with the carrier (25).In a study observed that wounds in 48 % of diabetic patients treated with tretinoin were fully recovered. Also, wounds area and depth of wounds after treatment with tretinoin significantly were reduced in comparison with control group. This study showed the short-term topical application of tretinoin accelerates recovery of diabetic ulcers (19). Retinoic acid can bind to nuclear receptors (retinoic acid receptor, RAR). Moreover, retinoic acid binds to intracellular retinoic acid proteins, likely involved in the adjustment of the cytosolic condensation of retinoic acid. The retinoic acid-receptor complex has a high affinity for binding to retinoic acid-responsive elements (RAREs) on DNA and induces the expression of genes during keratinocyte differentiation. A lack of vitamin A induces alterations in the keratinization of the epidermis due to a down regulation of genes involved in the expression of keratinocytes differentiation markers (26). Thus all reports mentioned the above confirm our findings. Also suggested that rose reduces inflammation, especially in the neck area (27). In a study conducted by Ulusoy and colleagues, it was demonstrated that of the rose oil has strong antimicrobial activity against Escherichia coli, Pseudomonas aeruginosa, Staphylococcus Violaceum Chromobacterium and the species of bacteria of Erwinia Carotovora (28). Their study can be considered as consistent with the present study because one of the factors which may prolong the process healing is the function of microorganisms such as the bacteria and fungi which in some cases, without inducing an obvious clinical sign and only in the subclinical form involve in the process of wound healing and reduce the rate of healing. Another study showed antibacterial effects of Rose extract against three species of bacteria of Xanthomonas Axonopodis (29). Another study showed significant antibacterial effects of rose leaves extract similar to the above study (30). Therefore, the results of the above studies can be in agreement with the present study.
Conclusion
It can be concluded from these findings that using a combination of retinoic acid and hydro-alcoholic extract of Rosa damascena mill can accelerate wound healing in diabetic animals. Therefore, this combination may be a good candidate to diabetic wounds healing.