Introduction
Catharanthus roseus (L.) G. Don (formerly Vinca rosea L., Apocynaceae) is commonly known as the Madagascar periwinkle. It is a perennial ever green herb, 30-100 cm tall originally native to the island of Madagascar but now widely dispersed in the tropics (1). The significance of this plant is its ability to synthesize a wide range of terpenoid indol alkaloids with medicinal values. These compounds have a broad application in the treatment of leukemia in children, and lymphocytic leukemia, Wilkins’s tumor, neuroblastoma and reticulum cell sarcoma, Hodgkin’s disease besides lymphosarcoma, choriocarcinoma (2). Except alkaloids, other natural compounds in C. roseus have been less investigated (3).
The genus Agrobacterium is gram-negative soil bacterium and belongs to the family Rhizobiaceae. Agrobacterium rhizogenes is the best known species, it can infect plants and cause abnormal proliferation of plant cells at the site of infection which is manifested by the appearance of adventitious roots called hairy roots (4). The transformation process depends on the presence of large size plasmid, 200-800 kilo base (kb) in the bacterium called root inducing (Ri) plasmid (5). These plasmids carry one or more DNA fragments called transferred-DNA (T-DNA). The T-DNA contain many genes, these genes are the four rol (root locus) genes, rolA, rolB, rolC and rolD. To identify the contribution of each T-DNA gene to the induction of hairy root disease (6). It has been reported that A. rhizogenesrol genes enhance the biosynthesis of certain groups of secondary metabolites in transformed plant cells. Of these genes, rolB is apparently the most powerful induce of secondary metabolism and rolB has the morphogenic effects similar to auxin-mediated effects (7).
Oxidative stress occurs as the result of an imbalance between the internal production of free radicals and the antioxidant defense mechanisms of living organisms (8). When the level of free radicals is increased and also when both the enzymatic systems and low molecular antioxidants are not sufficient to protect the organism, free radicals formed in excess induce cellular damage, which contribute to the development of diseases well as the spoilage of food products (9). Antioxidant enzymes such as catalase, glutamine peroxidase, and superoxide dismutase act to reduce the cellular level of free radicals. However, antioxidant supplementation is often necessary to limit the deleterious effects of the excess free radicals which have been formed. Synthetic antioxidants such as butylated hydroxy toluene (BHT) are being used in food industry to delay the oxidation of lipids. However, because of the tumorigenic potential and other side effects of chemical preservatives (10, 11), it is particularly important to replace them with herbs and natural antioxidants. Thus, plant tissue culture is being considered for producing valuable metabolites in significant quantities (12). Among the natural products with antioxidant properties, phenolic compounds have been extensively studied. Because these compounds neutralize free radicals and inhibit lipid oxidation, they inhibit the formation of toxins such as malondialdehyde (MDA). Such phenolic compounds are particularly useful in the preservation of food products and in the pharmaceutical industry. Additionally, these phenolic compounds directly promote health and well-being through disease prevention (13). Gallic acid is a type of phenolic acid with chemical formula C6H2(OH)3COOH with antioxidant, anti-inflammatory, antifungal and antitumor properties (14).
Previous studies have shown that aqueous extract of the root of C. roseus has good antioxidant properties (15, 16). However, because the root growth is limited, and that the root has pharmaceutical values, almost all roots are used in cultures to extract alkaloids. Therefore, plant tissue engineering and tissue culture are necessary to increase the capacity of the plant to produce secondary metabolites in greater quantities. Earlier studies indicated that the calli produced from the tissue culture of other plants can be a good source of useful secondary metabolites such as flavonoids (17). The hairy root culture is as an important resource of valuable secondary metabolites owing to rapid growth, short doubling time, and the dependable synthesis of chemical compounds (18). In the past two decades, considerable attention has been paid to the synthesis of these valuable hairy root compounds. The employment of cell suspension cultures from C. roseus has greatly expanded the production and enhanced the stability of secondary metabolites. In recent decades, this technique has extensively been used to produce more effective alkaloids and anti-cancer drugs from C. rosus (19). Continuing to research the anti-oxidation potential of these hairy roots can lead to other, more efficacious, applications of this transgenic product (20). To the best of our knowledge, no other report was found on the antioxidant potential of plant hairy roots and callus, and also their phenolic content. The present study assessed the antioxidant properties of transgenic hairy roots and leaf callus of C. roseus by measuring the free radical scavenging activity, reducing power, the ability to inhibit lipid peroxidation, and the capacity to inhibit the formation of MDA, and compared these results with those obtained for the root and leaf.
Experimental
Plant material and process hairy roots and callus calture
Plant material
Seeds from the roseus varieties of C. roseus were sterilized for 60 s with 70% ethanol and then for 15 min sodium hypochlorite (2 % available chlorine) followed by three washing with sterile distilled water and cultivated in pots inside a greenhouse. A sample of the plant was identified and a voucher specimen (6559-THE) was deposited in the herbarium, at the College of Pharmacy, Tehran University of Medicinal Science, Tehran, Iran. Explants were prepared from the young leaves (4-5 cm2, 2 months old) and used for hairy roots and callus induction (temperature 25-30 ºC and air humidity about 80%).
Agrobacterium rhizogenes suspension preparation
Agrobacterium rhizogenes strain ATCC 15834 was provided for transformation from the National Research Center for Genetic Engineering and Biotechnology of Iran that each cell of this bacteria harbors a Ri plasmid that involved in the root induction process; the root loci (rol) genes located in the TL region. The bacterial strain was cultivated in a new solid culture medium of LB (10 g L-1 Bactotrypton, 5 g L-1 yeast extract, 10 g L-1 sodium chloride plus agar 10 g L-1 in the acidity of 7, 50 mg L-1, rifampicin) at 28 ºC. A colony of grown bacteria was added to 5 mL liquid LB medium at 28 ºC insert on 180 rpm for 24 h in the dark. One mL of culture suspension was added to 30 mL of the same medium and shaked for 24 h, and the concentration of bacteria was adjusted to 0.4 - 0.6 by spectrophotometer (UV/Vis T90 PG instrument) at 600 nm wavelength (21).
Induction and establishment of hairy roots culture
Leaves obtained from young leaves of a 2 months old were used explants, each explants was infected by injection of A.rhizogenes strain ATCC 15834 suspension. In order to infect by injection, 5 µL of bacteria suspension were injected in to the dorsal vein of each leaf via insulin syringes angled at one or two positions. The leaf explants were blotted after 30 min with a sterile filter paper (to remove excess Agrobacterium) and transferred to petri dishes containing solid MS medium. Petri dishes were kept in darkness at 26 ºC for 48 h, and the samples were transferred to the MS medium (no hormone, 3 % sucrose, 500 mg L-1 cefotaxime and 0.8 % agar). Hairy roots appeared at the wound sites 10 - 15 days after infection, were allowed to grow about 2 cm, and were transferred to the solid MS medium supplemented with cefotaxime 500 mg L-1. After 4th subculture hairy roots on solid medium, The hairy roots were excised and immediately placed into 50 mL ½ MS liquid medium containing 3% sucrose and 500 mg L-1 cefotaxime in 250 mL Erlenmeyer flasks on a 60 rpm shaker (22). To ensure a sufficient amount of hairy roots, a subculture was created once every 2 weeks. After 3 months hairy roots kept and used for extraction.
PCR analysis
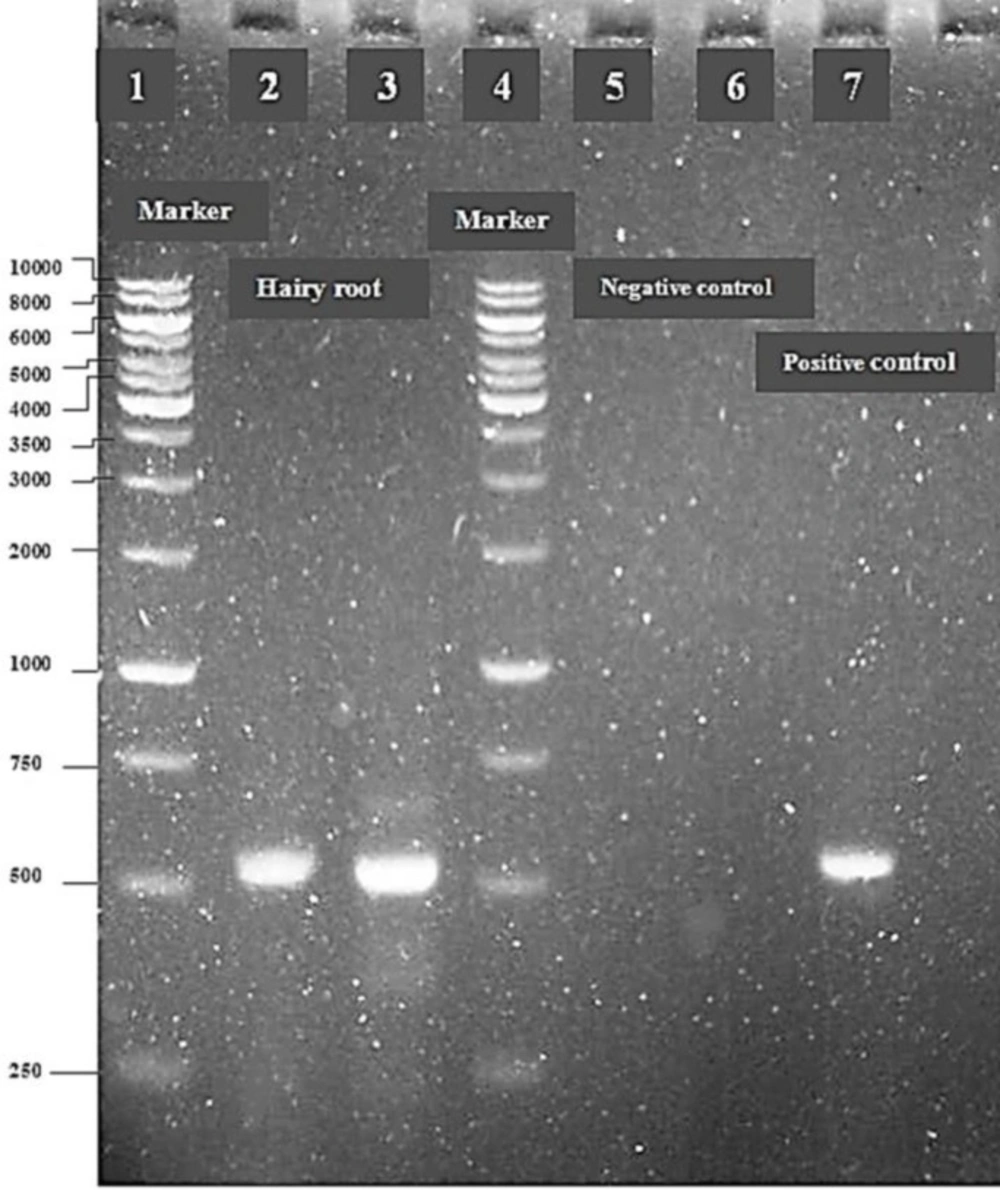
The presence of T-DNA in the hairy roots was by polymerase chain reaction (PCR) and the rolB was used as a target for PCR analysis in C.roseus hairy roots. Hairy roots were made bacteria free by transferring into a fresh medium containing cefotaime every 14 days. The hairy roots were checked for A.rhizogenes contamination by culturing hairy roots sample on LB medium. After the removal of bacteria, Genomic DNA was extracted via the CTAB procedure from hairy roots as well as from roots and leaves of C. roseus (23). Each PCR reaction (20.0 µL) contained: standard PCR buffer 10x (MgCl2 15 mM, KCl 500 mM, Tris-HCl 100mM, pH 8.3) 2.0 µL, dNTP Mix (10 mM) 0.5 µL, forward primer (10 µM) 1.0 µL, Reverse primer (10 µM) 1.0 µL, target DNA (50ng µL-1) 1.0 µL, Tag DNA polymerase 0.2 µL. Amplification conditions were: 35 thermal cycles, initial denaturizing at 94 ºC for 1 min, primer annealing at 57ºC for 1 min, and primer extension at 72°C for 1 min. The sequences of the two primers used to amplify a fragment of the rolB gene were: 5´-ATGGATCCCAAATTGCTATTCCCCACGA-3' (forward primer) and 5'- TTAGGCTTCTTTCATTCGGTTTACTGCAGC-3' (reverse primer) (24). Ladders 1 and 4 were used as standard marker (1 kb). The amplified product was run on 1.2% (w/v) agarose gel at 100 V to separate the DNA fragments.
Induction of callus culture
Plant leaves were sterilized for 60 s with 70 % ethanol and then for 15 min sodium hypochlorite (2 % available chlorine) followed by three washing with sterile distilled water and cut into pieces of 0.4 - 0.6 cm2 and were used for preparing the explants. The explants were transferred to petri dishes containing MS medium and 2, 4-Dichlorophenoxyacetic acid (2, 4-D) under sterile conditions. The cultures were maintained under a photoperiod (16 h/8 h light/ dark) at 26 ± 2 °C (25). To ensure a sufficient amount of callus, a subculture was created once every 2 weeks. After 45 days calli kept and used for extraction.
Extraction
To study the antioxidant composition, the plant material was dried in an oven at 40 °C for 72 h and ground by a grinder (IKA, Germany). Extraction was conducted little modified Sun et al. (2007) method with three replications (26). Briefly, a powder (200 mg) of each sample was extracted by 20 mL of ethanol 80 %, while being stirred at the temperature of 40 °C for 2 h. After this period, the mixture was filtered and the residue was remixed with the same solvent mixture ratio and the operation was repeated for 2 h. The filtered solutions of both stages were mixed together. The extracts were evaporated by using a rotary evaporator (Heidolph 4001, Germany) at 40 °C and were dried by a freeze dryer (Cherist 1-4 LD, UK). The ethanol extracts of samples were kept at -50 °C.
Antioxidant composition
Total phenolic content (TPC)
Total phenolic compound content was determined by the Folin-Ciocalteu method (27). In this way, 1 mL ethanol 80% was added to 10 mg dried extract powder of each samples and stirred. 200 µL diluted folin reagent (1:10) was added to 40 µL of this extract and the mixture was shaken. After 8 min, 600 µL sodium carbonate 7.5 % was added. After mixing the solution was diluted to 4 mL with ultra-pure water and mixed. After 90 min incubation at 23 °C the absorbance of samples was measured using spectrophotometer at 760 nm by spectrophotometer (UV/Vis T90 PG instrument Ltd). The total phenolic content was expressed as tannic acid equivalent in microgram per miligram of the extract using a standard curve generated with tannic acid (Merck, Germany).
Total flavonoid content (TFC)
Total flavonoid content of the extracts was measured spectrophotometrically using aluminum chloride as described by Zhishen et al. (1999). In this way, 500 µL ethanol extract (10 mg mL-1) of different plant organs was added to 2 mL double distilled water. Then, 150 µL sodium nitrite 5 % was added. After 6 min, 1 mL sodium hydroxide 1 M was added. The total volume of tube was adjusted to 5 mL with distilled water. After 15 min, the absorption of samples was read using spectrophotometer at 510 nm (28). The total flavonoid content was expressed as catechin equivalent in microgram per miligram of the extract using a standard curve generated with catechin (Sigma-Aldrich, USA).
Gallic acid content (GAC)
The chromatographic analysis was carried out on a HPLC system (Knauer, Germany) equipped with a K-1001 pump and a UV-Vis detector (K-2600) at 271 nm, column: C-18 (4.6×250 mm, 5 µ particle size). An isocratic mobile phase of methanol: ethyl acetate: water (25:50:70 v/v) with an elution volume of 0.7 mL min-1 was selected. 20 µL of each extract was injected. The column temperature was maintained at 37 °C. Identification of gallic acid in samples was based on retention time in comparison with standard (pure gallic acid). The quantification was carried out using the external standard method. The solution of pure gallic acid (Merck, Germany) at various concentrations (1-5 µg mL-1) was injected into the HPLC system and the calibration curve was established. The concentration of gallic acid in each sample was calculated from peak area according to calibration curve (29). The calibration curve was linear with r2=0.992.
Antioxidant activity
Free radical scavenging activity (DPPH assay)
The 1, 1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging activity was assessed according to the method of Brand-Williams et al. (1995). The absorption using spectrophotometer was measured at 517 nm after 60 min (30). Free radicals not treated with extracts served as controls. The following equation was utilized to calculate the percentage of free radical scavenging: Scavenging activity% = (1 - Asample/A0) × 100.
Where Asample is the absorbance in the presence of extract or positive control, while A0 is the absorbance in the absence of extract (31). The half maximal inhibitory concentration (IC50) was defined as the concentration of the sample, required for scavenging 50% of the DPPH free radicals (32).
Ferric reducing antioxidant power (FRAP assay)
The reducing power of ethanol extract was measured according to the method of Ferreira et al. (2007). In this way, 1 mL different amount of each ethanol extracts (6.25 to 400 µg mL-1), 2.5 mL phosphate buffer 0.2 M (pH = 6.6), and 2.5 mL potassium ferro cyanide solution (10 mg mL-1) were mixed together and incubated at 50 ºC for 20 min. 2.5 mL trichloroacetic acid (Merck, Germany) 10 % was added to the mixture and was centrifuged at 3000 rpm for 10 min. 2.5 mL the supernatant was mixed with 1 mL distilled water and 0.1 mL ferric chloride (1.0 mg mL-1), and then the absorbance was measured at 700 nm (33). Butylated hydroxytoluene (Sigma-Aldrich, USA) was used as positive control. Reducing capacity of the samples was calculated by the following formula: Relative reducing effect % = [Asample - Amin/Amax - Amin] × 100
Where, Asample is the absorbance in the presence of extract or positive control, while Amin is the minimum absorbance in the test and Amax is the maximum absorbance (34).
Lipid peroxidation inhibitory activity (FTC assay)
The effect of ethanol extracts on lipid peroxidation was determined by the ferric thiocyanate (FTC) method (35). Different extracts (4 mg) were mixed with 4 mL of absolute ethanol, 4.1 mL linoleic acid (2.51 %, v/v) in absolute, 8 mL phosphate buffer (0.05 M, pH 7.0) and 3.9 mL distilled water. The mixture was placed at 40 °C in the dark. 0.1 mL this solution was then mixed with 9.7 mL ethanol 75 % and 0.1 mL ammonium thiocyanate (30 % w/v). Three minutes after adding 0.1 mL ferrous chloride (20 mM), the absorbance was measured at 500 nm in a spectrophotometer (UV/Vis T90 PG instrument Ltd). This step was repeated every 12 h until control reachs its maximal absorbance value. The mixture without added sample was used as a control. The inhibition of linoleic acid peroxidation by ethanol extracts was calculated as: Inhibition% = 100 - [(absorbance increase of the sample/absorbance increase of the control) × 100] (36).
Malondialdehyde inhibitory activity (TBARS assay)
The effect of ethanol extracts on the formation of MDA was determined by the thiobarbituric acid reactive substances (TBARS) assay (37). 1 mL each extract on the final hour (120 h) of the FTC assay was added to 1 mL aqueous thiobarbituric acid 0.67%. After boiling for 10 min, the tubes were centrifuged at 3000 rpm for 10 min. Absorbance of the supernatant was measured at 532 nm in a spectrophotometer (UV/Vis T90 PG instrument Ltd). The malondialdehyde scavenging activity was calculated according to the following equation: Inhibition% = (1 - Asample/A0) × 100
Where, Asample is the absorbance in the presence of extract or positive control, while A0 is the absorbance in the absence of extract (38).
Statistical analysis
The experimental results are expressed as mean with standard error. All measurements were taken in triplicate. The data were analyzed by an analysis of variance (P 0.01) and the means were compared by Duncan’s multiple range tests. The half maximal inhibitory concentration (IC50) values were calculated from a linear regression analysis. The statistical analysis was performed by Statistical Package for the Social Sciences version 22.
Results
Hairy roots and callus induction
The hairy roots were obtained after 10-15 days past transformation of C. roseus with A.rhizogenes strain ATCC 15834. The transformation efficiency was 3 % in co-cultivated explants (leaf) with bacteria suspension by injection method. The hairy roots were elongated and branched after growth (Figure 1). The transformed hairy roots was confirmed through PCR analysis by the presence of rolB. PCR products amplified with rolB primers were detected in the hairy roots and positive control (fragment with 500 bp). These results indicate that the rolB gene (500 bp), from the T-DNA of the A. rhizogenes Ri-plasmid, was integrated in to the genome of C. roseus hairy roots (Figure 2). No band was formed in the wells, which corresponded to the control (DNA extracted from natural roots and leaves, bands 5 and 6). Leaf calli were induced after two weeks with frequency of induction 90 % and reached full growth after 45 days (Figure 3).
Stages of hairy roots inoculation in C.roseus leaf and growth of these roots in MS culture medium A: Plant young leaf, B: Hairy root inoculation in leaf (one month after inoculation), C, D and E: Growing hairy roots in ½MS medium liquid (six, seven and eight weeks, respectively, after inoculation
Stages of leaf explants preparation, inoculation and C. roseus callus growth in medium containing dichlorophenoxyacetic acid (2, 4-D) A: Young leaves for preparation of explant, B: Cutting leaf into 4-5cm2 pieces C: Two weeks after explant culture in culture medium, D: Magnified image of an explant transforming into callus, E: Formed callus, six weeks after culture in MS medium
PCR analysis of C.roseus hairy roots for rolB transgenesis with A.rhizogenes ATCC 15834. Molecular weight marker (lanes 1 and 4, 1kb), transgenic hairy roots (lanes 2 and 3), genomic DNA from normal root and leaf culture separately (negative control, lanes 5 and 6), plasmid genome (positive control, lane 7
Antioxidant composition
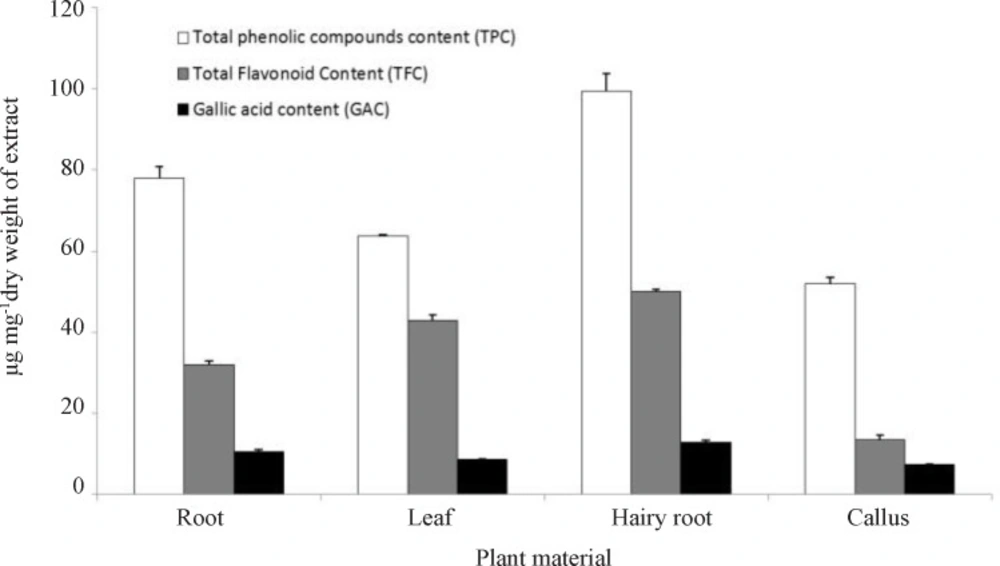
The results showed the significant difference between of phenolic content for dried ethanol extract of root, leaf, hairy roots and callus (Figure 4). Phenolic content was higher in hairy root (99.38 ± 4.38) than in root (78 ± 2.79), leaf (63.63 ± 0.42) and callus (51.84 ± 1.69), equal to µg of tannic acid per milligram of dried weight of extract according to the standard curve of y = 0.000492x + 0.04819, r2 = 0.973. According to the equation (y = 0.00289x - 0.0076, r2 = 0.989), flavonoids content was higher in hairy root (50.1 ± 0.35) than in root (32.02 ± 0.91), leaf (42.9 ± 1.34) and callus (13.46 ± 1.2), equal to µg of catechin per mg of dried weight of extract. Gallic acid content was higher in hairy root (12.86 ± 0.37) than in root (10.55 ± 0.37), leaf (8.52 ± 0.19) and callus (7.28 ± 0.19), µg gallic acid per mg of dried weight of extract according to the standard curve of y = 34385.7x + 845900, r2 = 0.992 by HPLC.
Antioxidant activity
Free radical scavenging activity
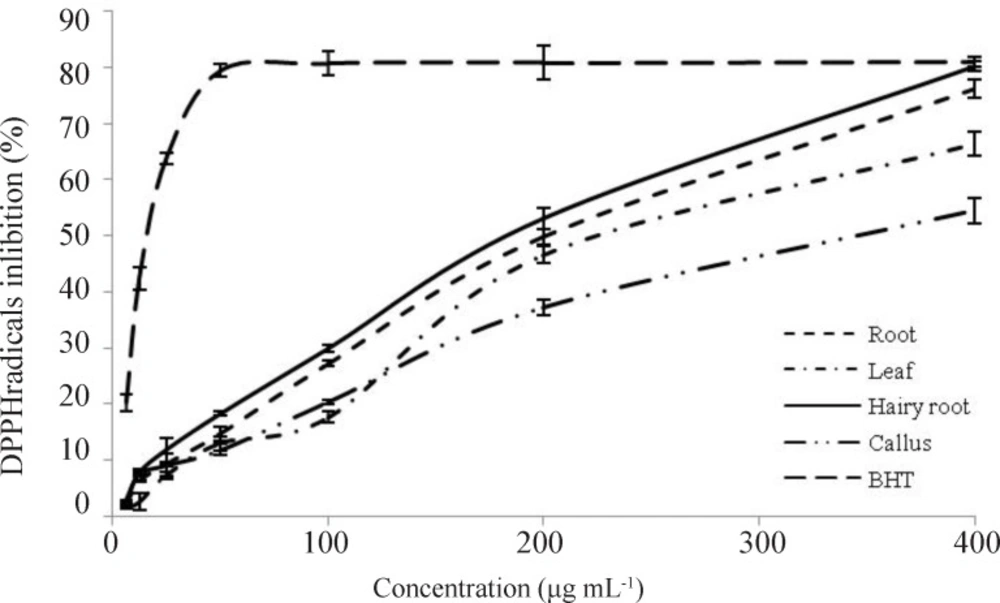
According to figure 5, percentage of free radical scavenging at concentration of 6.25 to 400 µg mL-1 of ethanol extract samples compared to standard antioxidant showed that increased concentration of ethanol extracts led to increased inhibition of free radicals, and concentration of standard antioxidant in excess of 50 µg mL-1 had no significant effect on increasing inhibition of free radicals. The hairy roots extract showed the best performance in DPPH free radical trapping activity compared with C. roseus root, leaf, and callus. The ethanol extracts of hairy root, root, leaf, and callus at the concentrations of 221.22 ± 5.85, 238.9 ± 2.02, 277.95 ± 4.443 and 342.11 ± 2.67 µg mL-1, respectively, scavenged a half of DPPH radicals in contrast to standard antioxidants) 22.29 ± 0.4 µg mL-1(. At equal concentrations, the standard antioxidant showed superior radical scavenging properties. However, at higher concentrations, the extracts, particularly that of hairy root (400 µg mL-1), scavenged free radicals as efficiently as the standard antioxidant (BHT).
Ferric Reducing antioxidant power (FRAP assay)
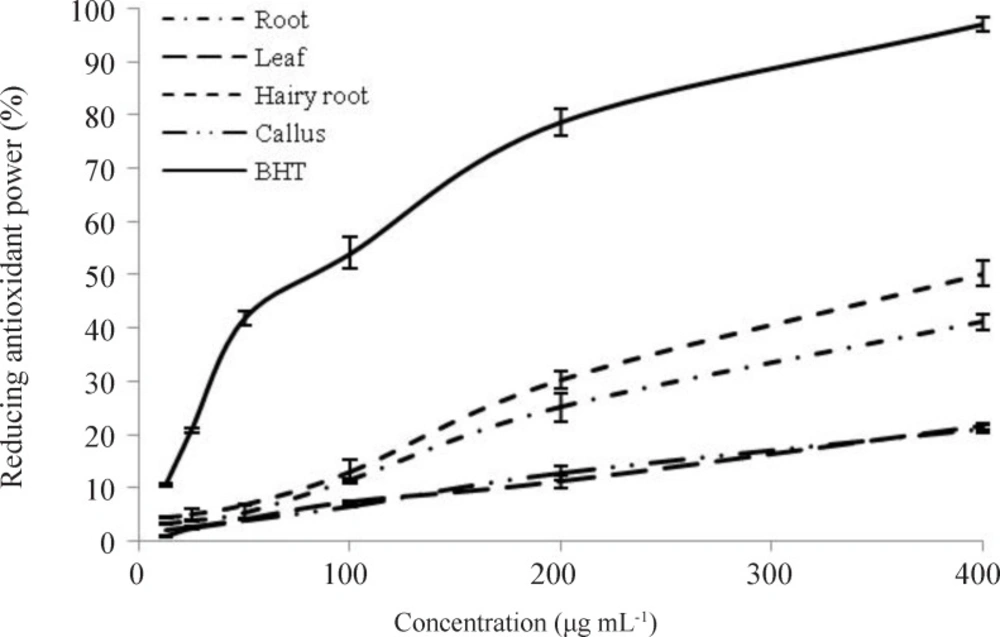
Reducing power of ethanol extract samples at concentration of 6.25 to 400 µg mL-1 showed that increased concentration of extracts led to increased percentage of Fe (III) reduction (Figure 6). Hairy roots extract showed greater reducing power. The ethanol extracts of hairy root, root, leaf, and callus, at concentrations of 393.07 ± 2.8, 407.70 ± 4.75, 745.68 ± 6.46, and 961.71 ± 25.1 µg mL-1, respectively, were able to reduce half of the Fe3+ into Fe2+ ions in contrast to the standard antioxidant (114.33 ± 1.18 µg mL-1 ).
Lipid peroxidation inhibitory activity (FTC assay)
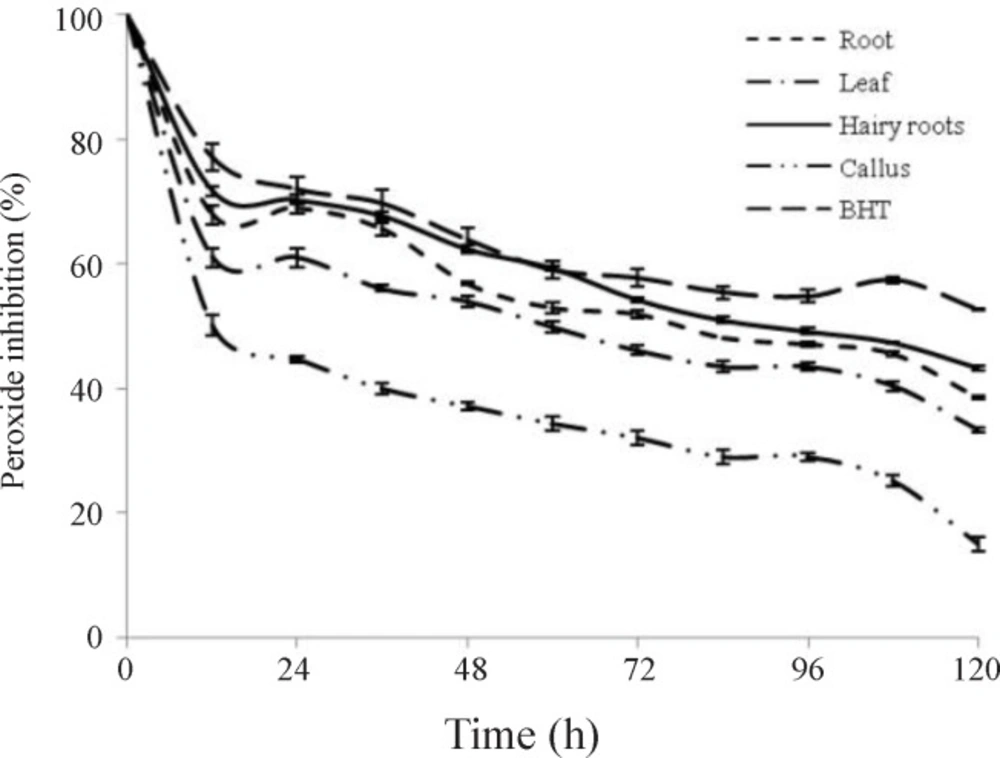
FTC assay measures the amount of produced at the initial stage of lipid oxidation. According to figure 7, percentage of inhibition of linoleic acid peroxidation at final concentration (200 µg ml-1) of ethanol extract samples and standard antioxidant at 37 °C showed that all extracts are capable of inhibiting linoleic acid peroxidation at maximal absorption of the control (after 108 h), the extent of inhibition of linoleic acid peroxidation by extracts followed the orders: hairy roots > root > leaf > callus. Hairy roots extract showed superior antioxidant properties than other extracts. After 12 h, hairy root extract and BHT inhibited linoleic acid peroxidation 71.6 ± 0.84 % and 77.14 ± 0.17 %, respectively, whereas the extent of inhibition by these extracts after 108 h was 47.25 ± 0.2 % and 52.65 ± 0.17 %, respectively. After 12 h, ethanol extracts of root, leaf, and callus inhibited reaction by 67 %, 60 %, and 50 % respectively, and 108 h later, their inhibitory power reached 45 %, 40 %, and 25 %, respectively. The results also showed increased peroxide in test tubes with increasing time.
Malondialdehyde inhibitory activity (TBARS assay)
The results also revealed that the ethanol extracts potently inhibited formation of MDA (Table 1). The performance of the hairy root extract was superior. The hairy root extract inhibited the formation of this toxic substance by 83 %, whereas the extent of inhibition by the standard antioxidant was 87 % (Table 1).
| Plant material | Malondialdehyde inhibition (%) |
|---|---|
| Root | 78.68±0.54 |
| Leaf | 42.96±0.92 |
| Hairy roots | 83.24±0.51 |
| Callus | 35.62±1.29 |
| BHT | 87.47±0.55 |
Relationship between antioxidant composition and antioxidant activity
A strong relationship was observed between phenolic, flavonoid and gallic acid contents and antioxidant power of the plant in inhibition of free radicals, reducing power and inhibition of MDA formation. Pearson coefficients were applied in determining the relationship between antioxidant composition (phenolic, flavonoid and gallic acid contents) and antioxidant capacity of the extracts based on reducing antioxidant power, DPPH and MDA inhibition (Table 2).
Significant at p ≤ 0.01,
Significant at p ≤ 0.05
Discussion
Various species of bacteria can transfer genes to higher plants (5). A. rhizogenes is one of the most widly studied among them. It infects the plant cell and leads to the formation of hairy roots (39). A. rhizogenes ATCC 15834 is one of the most common strain used for hairy root induction. Several other studies have reported on hairy roots induced by this strain (40, 41). Pratap Chandran et al. (2008) reported exit points of hairy roots which induced by A. rhizogenes strain ATCC 15834 from the stem, hypocotyl and epicotyl of five host plant, were after 10 days (42), which was confirmed in the present study. Recently, hairy roots applied for production of secondary metabolit (18). According to reports, rolB gene is able to increase biosynthesis of secondary metabolites (7). Thus, this gene is expected to stimulate synthesized secondary metabolites in transformed hairy roots. Studies have, in fact shown that secondary metabolites are produced faster and more abundantly in hairy roots than in other normal parts of the plant. Karthikeyan et al. (2010) reported that the total alkaloid content was 1 % in a leaf, 2–3 % in root, and up to 9% in hairy roots (3). Some studies show that alkaloid synthesis routes are closely allied with the synthesis of phenolic compounds (43). Among these phenolic compounds, a higher level of gallic acid was found in hairy roots versus roots and leaves, which signals greater antioxidant properties.
Due to limitation in culture and increased demand for secondary metabolites, alternative natural sources were considered, such as hairy roots and callus culture, which was confirmed by Bolda et al. (2011), reporting that callus can be considered as a source of antioxidants (44). Shahidi and Zhong (2005) reported that many secondary metabolites, such as phenolic compositions, contain antioxidant properties (45), thus this study aimed to investigate and compare the antioxidant potential. This study aimed to investigate and compare the antioxidant potential of transgenic hairy roots from the leaves of C. roseus infected with A. rhizogenes strain ATCC 15834 and leaves callus of this plant with natural root and leaves of this plant.
Prostus et al. (46) reported that the plant leaves have high phenol content. Mustafa and Verporter (2007) reported that the plant contains considerable amounts of phenolic compounds as well as alkaloid (47). A report by Sroka and Cisowski (2003) cites some phenolic acid compounds in the plant, such as gallic acid (48). These reports confirm the presence of phenolic compounds in the plant. Shahidi and Zhong (2005) believe phenolic compositions are one of the best natural sources of antioxidants, which play an important role in protecting tissues against oxidizing free radicals of oxygen and other active strains (45). This property in plants depends upon the amount of each polyphenolic compounds.
DPPH free radical inhibition method that relies on hydrogen-donating activity illustrates an important mechanism of antioxidant activity of phenolic compositions. The present study results showed that ethanol extracts are capable of inhibiting this radical. Although root and leaf were appropriately capable of inhibiting this radical, transformed roots had greater inhibition power. Even though the present study is a new report on inhibition power of free radicals by hairy roots of this plant, previous reports on its root (15), and leaf (16) confirm inhibition of free radicals and hydrogen-donating capability of ethanol extracts. Transformed hairy roots of the plant at concentration of 400 µg mL-1 had similar performance to synthesized antioxidants.
Fe (III) reducing is often used as an indicator of electron-donating activity, which is an important mechanism of phenolic antioxidant action (49). Amount of Fe2+ complex can be then be monitored by measuring the formation of Perl's Prussian blue at 700 nm.
The present study results showed that all ethanol extracts were capable of electron donation. Although extracts in hydrogen donation activity in DPPH free radical scavenging test were able to neutralize half radicals by hydrogen donation at lower concentrations; hairy roots were capable of higher electron donation compared to other extracts. Although the present study provides a new report on hydrogen donation power of extracts of this plant, Weremczuk-Jezyna et al. (2013) reported significantly stronger hydrogen donation activity than electron donation activity in hairy roots of Dracocephalummoldavia, which agrees with the present study results (50). Asan Uzosaglam and Karacoca (2014), reported high antioxidant capability of Glycyrrhiza glabra L., and extracts of root and aerial parts of this plant at concentrations 582.14 and 432.63 µg mL-1 respectively were able to convert half Fe III ions to Fe II ions (51). Yet, in the present study, extracts of hairy root and C.roseus root were stronger than G.glabra.
Lipid peroxidation is a free-radical-mediated chain of reactions that, once initiated, results in oxidative deterioration of polyunsaturated lipids. Once formed, the unstable lipid peroxides, degrade rapidly into a variety of products. These reactions can be initiated or facilitated by toxic products, including endoperoxides and aldehydes. Antioxidants donate one hydrogen atom to peroxperyl radicals, converting them into non-radical species (52). In this study, ethanol extracts had similar function to synthesized antioxidant in inhibition of linoleic acid peroxidation, and extract of hairy roots prevented this process more than other extracts. The present study is a new report on capability C.roseus ethanol extract. The results showed reduced capability of extracts and synthesized antioxidant in inhibition of peroxide radicals with increasing time, which suggests accumulation of these radicals in reaction medium.his finding, agrees with Mathew and Emili Abraham report (2006) on reducing trend of lipid peroxidation of Cinnamomum verum leaf extract, and synthesized antioxidant with time in the present study, and also reported C.verum leaf extract 24 and 144 h after experiment was able to inhibit 80 % and 50 %, respectively of linoleic acid peroxidation process (53), which is similar to performance of hairy root extract of this plant.
Malondialdehyde has been used to assess the extent of lipid peroxidation (52). The injury mediated by free radicals can be assessed by measuring the amounts of conjugated dienes, MDA, and other products. Compounds capable of inhibiting the formation of this colored complex are considered antioxidants. Extracts of plant material used in this study showed the ability inhibit the formation of MDA. Compared to other extracts, hairy roots of the plant inhibited greater percentage of MDA, and showed a very similar performance to synthesized antioxidant. To our knowledge, this is the first report describing the ability of C.roseus extracts to inhibit the formation of MDA. Rezazadeh et al. (2011) in a study of MDA inhibition methanol extract of Momodia charantia fruit reported that this extract inhibited 32.5 % of MDA by the end of experiment (8th day) (37). But, C. roseus ethanol extract had stronger performance than this plant.
Phenolic compositions act like antioxidants because they donate hydrogen or electron to free radicals (54). Hydrogen or electron donation to free radicals and formation of a complex between the lipid radical and the antioxidant radical (free radical acceptor) are among the chemical mechanisms that empower antioxidants to inhibit free radicals (55). This finding was confirmed in the present study by the strong relationship between phenolic compositions and antioxidant activity
Conclusion
The results of the present study revealed that transformed hairy roots with C.roseusrolB gene had a considerable antioxidant power and antioxidant compositions compared to its root, leaf, and callus. The greater presence of phenolic compositions in transformed hairy roots can be attributed to rolB gene transfer to plant leaf. Hairy roots hydrogen donation power in free radical scavenging and also electron donation power as greater reducing power compared to other extracts led to considerable power of hairy roots in inhibition of lipid peroxidation process and prevention of toxic MDA formation. Although plant callus can be considered as a source of antioxidant, it did not have a high phenolic content or antioxidant power due to its short life in vitro. Therefore, from a pharmaceutical perspective, the extraction of their potent antioxidants from transformed hairy roots of the plant can be very beneficial in inhibiting free radicals and reducing the risk of degenerative diseases. Transformed hairy roots of the plant can be a rich source of natural antioxidants.