Introduction
Lung cancer is a leading cause of cancer death worldwide, in which amplification of oncogenes contributes to inflammation and tumorogenesis (1, 2). A vast array of mediators orchestrates inflammation signaling pathways destined to invasion, metastasis, and angiogenesis in lung cancer (-). Meanwhile, COX-2 is a key modulatory molecule engaged in the pathogenesis and development of the lung neoplasia (-). Consistently, growing evidences has emphasized COX-2 blockers as a promising therapeutic approach in a variety of malignancies, in particular lung tumors (-). Accumulating investigations have substantiated that Celecoxib exerts its effect partly through COX-2-independent mechanisms, including inhibition of Akt signaling (-). Akt is a crucial signaling molecule that delivers mitogenic and anti-apoptotic signals (17, 18). It is known that Akt promotes p53 degradation via MDM2 (19). p53 is a well-established tumor suppressor protein and key regulator of apoptotic responses, while MDM2 is a major modulator of p53 activity via binding to and thereby promoting its degradation (20). Thus, the consequent Akt blockade can affect p53. On the other hand, several lines of evidence have addressed functional crosstalk between p53 and COX-2 signaling pathway (11, 12 and -). Given these molecular interconnections stated above, we sought to investigate the effect of COX-2 signaling blockade on p53 and COX-2 expression pattern in A549 cells.
Experimental
Reagents
RPMI 1640 media, FBS (Fetal Bovine Serum), trypsin, penicillin, and streptomycin were purchased from Biosera (Austria). MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide] was purchased from Sigma (USA). Celecoxib was from Tehran University of Medical Sciences, Faculty of Pharmacy (Iran). Western blot detection kit and polyvinyl difluoride (PVDF) membrane were provided from Roche (Germany). Anti-FHIT antibody was from Abcam (USA). p53 and COX-2 antibodies were provided from cell signaling (U.S.A).MDM2 antibody was from Novus (USA). β-actin antibody was from Santa Cruz Biotech (USA). The rest of the reagents, including chemicals were from Merck (Germany).
Cell culture
Parental A549 cells were obtained from EACC. Cells were cultured in RPMI-1640 media with 10 % fetal bovine serum, penicillin (100 IU/mL), and Streptomycin (100 mg/mL) at 37 °C in a 5 % CO2 humid atmosphere.
Drug treatment
Cells were exposed to different concentrations of Celecoxib (10-100µM). Subsequently, treated cells were harvested within 24 and 48 h. Dimethyl sulfoxide(DMSO) (equal volume to added drug) was used as the vehicle control in all the experiments.
Cell viability assay
The effect of Celecoxib on cell viability was assessed using MTT method. Briefly, cells were seeded at 10,000 cells per well in 96-well plate and were treated with Celecoxib for 24 and 48 h. Subsequently, MTT solution was added at final concentration of 5 mg/mL and cells were incubated (37 °C, 5% CO2) for additional 4 h. The formazan crystals were dissolved using 60μL of DMSO and the absorbance was measured using micro plate reader (Anthos 2020, Austria) at 570 nm against690 nm. The cell viability was finally calculated as percentage of control group.
Western Blotting
After incubation period, cells were harvested into Laemli lysis buffer (Tris62.5mM, pH 6.8, dithiothreitol 50mM, sodium dodecyl sulphate (SDS) 2 %, Glycerol 10 % and Bromophenol Blue 0.25 % (w/v). Equal amount of protein samples were subjected to 12 % SDS–PAGE; and transferred to PVDF membranes. Membranes were then gently blocked with 1 % casein for 4h at room temperature and incubated with anti-p53 (1:1000), anti-COX-2 (1:1000), and anti-MDM2 (1/1000) antibodies overnight at 4 °C. Anti-β-actin antibody (1:2000) was used as internal control. The blots were then exposed to HRP-conjugated secondary antibody (1:10000) for 1 h at room temperature. Protein bands were revealed using chemiluminescence kit on X-ray film. The protein densitometry analysis was carried out using Image J software (USA) and expressed as Mean±SE of three independent experiments.
Statistical analysis
All of the experiments were conducted at least three times independently. Results were presented as Mean±SE. Multiple comparisons were carried out using One-way Analysis of Variance (ANOVA) followed by a Tukey–Kramer post hoc analysis. P values less than 0.05 were regarded as statistically significant.
Results
Celecoxib-induced growth inhibition
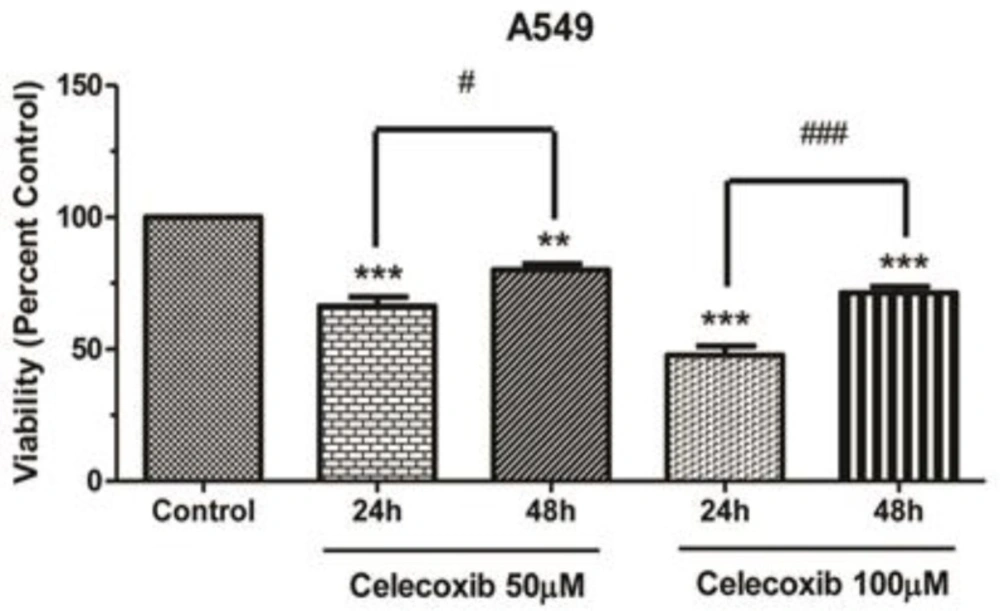
We examined the effect of different doses of Celecoxib (10-100µM) on A549 cell proliferation. The lower stimuli (10, 25µM) did not provoke significant growth inhibition at both time points compared to the corresponding control (data not shown). Hence, we investigated the effect of higher doses of Celecoxib on cell proliferation. As plotted in Figure 1, Celecoxib exerted marked growth inhibition upon 50,100µM within 24 h, whereas prolonged exposure interestingly increased cell viability compared to the corresponding 24 h (P values<0.05 and 0.001, respectively). These results imply that Celecoxib biphasically affects cell proliferation in a dose-and time-dependent manner.
Celecoxib-induced growth inhibition in A549 cells. Cell viability assay was performed in parental cells. Cells were seeded in 96-well plates and exposed to Celecoxib for 24h and 48h. Proliferation assay was carried out using MTT method. Results were calculated as percent control and presented as Mean±SE of three separate experiments (*p<0.05, **p<0.01, and ***p<0.001 compared to control
Celecoxib treatment enhances 53 expression
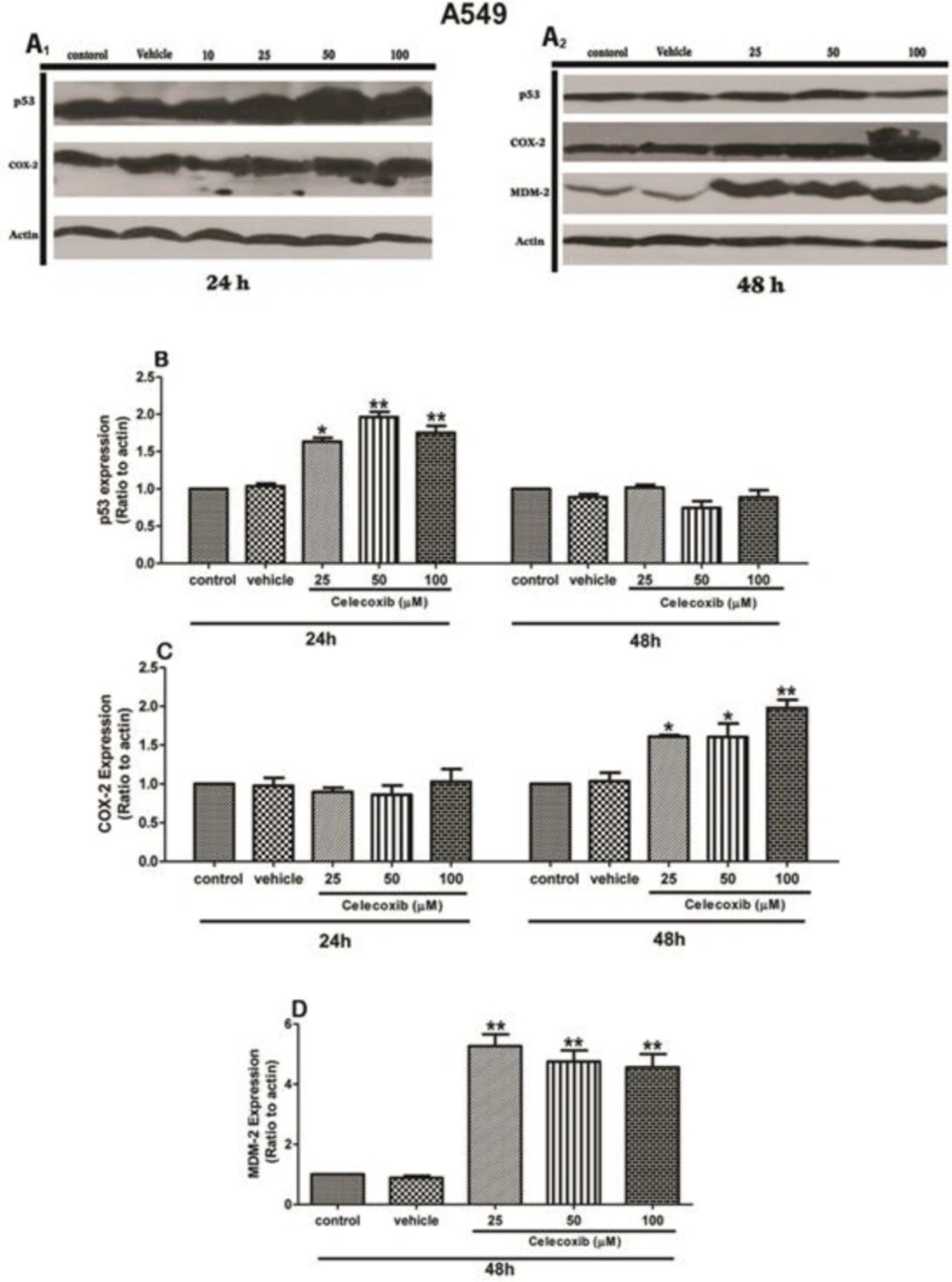
COX-2 is known to be an oncogene being interconnected with a number of tumor suppressors, such as p53. It has been appeared that COX-2 negatively regulates p53 expression. Consequently, we aimed to examine whether COX-2 signaling blockade can affect on p53 expression. In this respect, we evaluated p53 expression upon Celecoxib treatment in A549 cells within 24, 48 h. As depicted in Figure 2C, Celecoxib dose-dependently augmented p53 expression within 24 h (1.5-2-fold of control, P values <0.05, 0.01, respectively)which was returned to the control level at 48 h.These results express that Celecoxib modulates p53 expression in a dose- and time-dependent manner in wild-type A549 cells.
The effect of Celecoxib on p53 (B), COX-2 (C), and MDM2 (D) expression in wild-type A549 cells. Cells were treated for 24 and 48 h and harvested within the indicated time points. The protein expression profile was analyzed by western blotting. ß-actin was used as internal control. The protein expression was calculated as ratio to actin and presented as Mean±SE of three independent experiments (*p<0.05, **p<0.01, and ***p<0.001 compared to the corresponding control
COX-2 expression alterations in response to Celecoxib treatment
In our quest to understand the effect of Celecoxib on COX-2 signaling pathway, we exposed cells to Celecoxib and COX-2 expression alterations were assessed within 24 and 48 h. As demonstrated in Figure 2C, Celecoxib negligibly attenuated COX-2 level within 24 h in parental cells, whereas incubation within 48 h elevated COX-2 expression compared to the corresponding control (1.5-fold, P value<0.05). Such findings indicate that COX-2 signaling inhibition strictly depends on the timing of the stimuli which implies that Celecoxib treatment may not be a proper approach within longer incubation periods.
MDM2 is markedly induced following Celecoxib treatment
MDM2 is considered as the major protein modulating the expression of p53.Considering the effect of Celecoxib on parental cells at 48 h, we attempted to examine whether Celecoxib affects MDM2 expression at this time point. As depicted in Figure 2D, Celecoxib remarkably led to elevate MDM2 expression level within 48 h (5-fold of control, P value<0.01), implying COX-2 expression correlates with MDM2 signaling modulation in A549 cells.
Discussion
COX-2 signaling pathway has been addressed as an indispensable mediator in the pathogenesis of a variety of malignancies, in particular lung cancer (6, 7). Consistent with this notion, it has been appeared that COX-2 pathway blockade can be a desirable approach in the treatment of a range of cancer models, including the lung neoplasia (27). Previous experiments have delineated functional crosstalk between COX-2 and a subset of molecular targets, such as p53 (-). Considering these statements, the role of COX-2 inhibitors as a possible candidate to induce cell death through apoptotic machinery has been investigated in numerous reports thus far (23, 28).
In this respect, we aimed to examine the effect of Celecoxib on p53 expression and persued p53 signaling interaction with COX-2 and MDM2 pathways upon Celecoxib treatment in A549 cells. The cell viability assay has shown that Celecoxib caused a dose-dependent growth inhibition in wild-type A549 cells within 24 h. However, prolonged exposure to the drug conversely led to increase cell viability percent, suggesting that Celecoxib treatment biphasically modulates cell growth depending on the dose and timing of the treatment. In accordance with this, our western blot results have also demonstrated that Celecoxib enhanced p53 expression within 24h in parental cells in a dose-dependent manner (1.5-2-fold of control, P values <0.05, 0.01, respectively). It has been appeared that Akt is a self-evident repressor of the p53 expression through MDM2 and consistently, several studies have reported that Celecoxib can inhibit Akt signaling pathway hence, the resultant p53 up-regulation might be ascribed to disruption of Akt signaling induced by Celecoxib(-, 29). Although Celecoxib could increase p53 level within 24h, COX-2 expression was barely affected at this time point, indicating that Celecoxib augmented p53 expression, independently of COX-2 inhibition (Figures 2B, C). This result suggests p53 as a COX-2-independent molecular target. Considering this finding, we next sought to determine whether inhibition of COX-2 pathway affects prolonged p53 signaling. Paradoxically, Celecoxib exposure lowered p53 expression within 48h, so that p53 returned to the control level at this time point compared to the corresponding 24 h (Figure 2B). Coincidentally, Celecoxib strikingly could result in COX-2 induction at 48 h (Figure 2C).The resultant COX-2 elevation upon Celecoxib treatment can be attributed to p53 that can induce COX-2 expression through Ras/Raf/MAPK cascade according to the prior experiments(24). Accompanying this, MDM2 expression was markedly augmented at 48h, indicating a direct correlation between these signaling pathways (Figure 2D). Thus, it seems that p53 triggers a negative regulatory feedback which finely tunes the cellular homeostasis by manipulating the expression of both MDM2 and COX-2.From the clinical standpoint, such findings can point toward the possibility that Celecoxib treatment may not be a proper therapeutic strategy in lung cancer cells owing to its potential role in the activation of counter-regulatory pathways, resulting in the induction of crucial oncogenes, such as COX-2 and MDM2. In supporting this issue, a recent investigation has also substantiated that Celecoxib promotes cell invasion and confers cells resistant to chemotherapy through activation of MEK-ERK signaling cascade (30). Consistently, it has been appeared that ERK promotes tumorogenesis via MDM2 signaling axis (31).Conceivably, blockage of MDM2 and/or other contributing mediators induced by Celecoxib might be an effective approach to hamper Celecoxib-induced cancer promotion, although elucidating such assumptions demands further investigations. Taken together, such a scenario underscores intensive preclinical assessment before applying COX-2 inhibitors in the treatment of lung tumors.