Introduction
Nano-sized materials exhibit interesting properties (1) and present new opportunities for a wide range of studies and applications in various areas including physics (2), chemistry (3), biology (4), and medicine (5). Use of nanotechnology in various therapeutic sectors has revolutionized the field of medicine, in which nanostructures are used as biomedical tools for investigation (6).
Common anticancer drugs not only affect cancer cells but also influence normal cells and cause severe side effects. To achieve more effective cancer chemotherapy, it is essential to appropriately select anticancer drugs for the cancer cells. Since the complications of anticancer drugs include their impact on normal cells, it is preferred to utilize targeted drug delivery systems. Nanoparticulate drug delivery systems such as liposomes (7), polymers (8), nanoparticles (5), dendrimers (9), and carbon nanotubes (10) which contain anticancer agents have received a great deal of attention due to their unique accumulation behaviors in tumor sites. One of the best and ideal solutions for most of the serious problems in chemotherapy is application of drug-loaded NPs which have targeting functions (11). NP surface is decorated with different ligands that are delivered within cells through receptor-mediated endocytosis. Folate (FOL), as one of these end ocytosis, has been widely used owing to its many advantages such as small ligand size, convenient availability, low cost, relative simplicity and defined conjugation chemistry, high receptor affinity, absence of normal tissue receptor expression, and therefore high tumor tissue specificity (5, 9 and 12). As a glycosyl phosphatidyl inositol anchored cell surface receptor for folate, folate binding protein is over-expressed in some human tumors; however, it is widelylimited in normal tissues (13). Using folate decorated liposomes, micelles, and NPs of biodegradable polymers including poly-lactide-co-glycolide (PLGA), PLGA–polyethylene glycol (PEG), D-alpha-tocopheryl polyethylene glycol 1000 succinate (vitamin E TPGS) –folate conjugate, doxorubicin–PLGA–vitamin E TPGS conjugate, and PLGA-PEG-FOL conjugate as anticancer agents has raised cellular uptake and cell cytotoxicity of the formulated anticancer drugs (5, 9 and 10).
In attempts to investigate a suitable nanocarrier system, carbon nanotubes (CNTs) have attracted great attention in recent years (10, 14). Suitable functionalization is an important factor that affects cytotoxicity and performance of CNTs in biological systems (15).
In the present work, PEG-FA-conjugated SMWCNT@Fe3O4, which could be used as a multi-targeted drug nanocarrier for delivering an anti-cancer agent to cancer cells with the assistance of an external magnetic field, was prepared and characterized.
Experimental
Materials
Short MWCNTs (diameter: 30–50 nm, length: 0.5~2 μm, purity: >95%, and SSA: 200 m2/g) were purchased from Neutrino Nanotechnologies Co., Iran. Ferrous chloride tetrahydrate (FeCl2.4H2O, 99 %), ferric chloride hexahydrate (FeCl3.6H2O, 99%), sodium hydroxide (NaOH), concentrated nitric acid (HNO3, 68% v/v), and concentrated sulfuric acid (H2SO4, 98% v/v) were purchased from Merck Co. Ltd., Germany. Poly (ethylene glycol) bis-amine (NH2-PEG-NH2, MW: 1500), folic acid, N-hydroxysuccinimide (NHS), and dicyclohexylcarbodiimde (DCC) were obtained from Sigma (St. Louis, MO).
Methods
Functionalizing carboxylic acid on SMWCNTs
Briefly, 90 mL of concentrated H2SO4 was added to 1 g of raw SMWCNT under stirring. Subsequently, 30 mL of concentrated HNO3 was added drop-wise to the mixture under sonication. The resulting mixture was refluxed at 110˚C in an oil bath for 12 h. After dilution with 5-fold water, the solution was filtered through a paper filter (Whatman 42) to remove any unreacted bulk of CNTs. The added water was evaporated to almost reach the previous volume (120 mL) (10, 14-16).
Neutralizing the solution acidity by base
In the previous work, magnetization method of carbon nanotubes was explained, a summary of which is given below. The remainder acid could be neutralized with sodium hydroxide in 50 wt% aqueous solution to form sodium sulfate and sodium nitrate salts and water as the end products of the reaction. After reaching pH value up to 11, the mixture containing carboxylated SMWCNTs (c-SMWCNTs) was ready for the attachment of Fe3O4 nanoparticles (16).
Attaching Fe3O4 nanoparticles to c-SMWCNT
First, FeCl3•6H2O (420 mg) was dissolved in the mixture under sonication. Then, FeCl2•4H2O (153 mg) was dissolved in 6 mL deionized water and added drop-wise to the final mixture under vigorous stirring and nitrogen atmosphere at 80˚C. After aging for 15 min at the same temperature, the mixture was cooled down to room temperature under stirring. Then, the product was collected using a strong magnet and washed with deionized water for several times until reaching the pH value of about 7 (16-20).
Preparing PEG-FOL conjugate
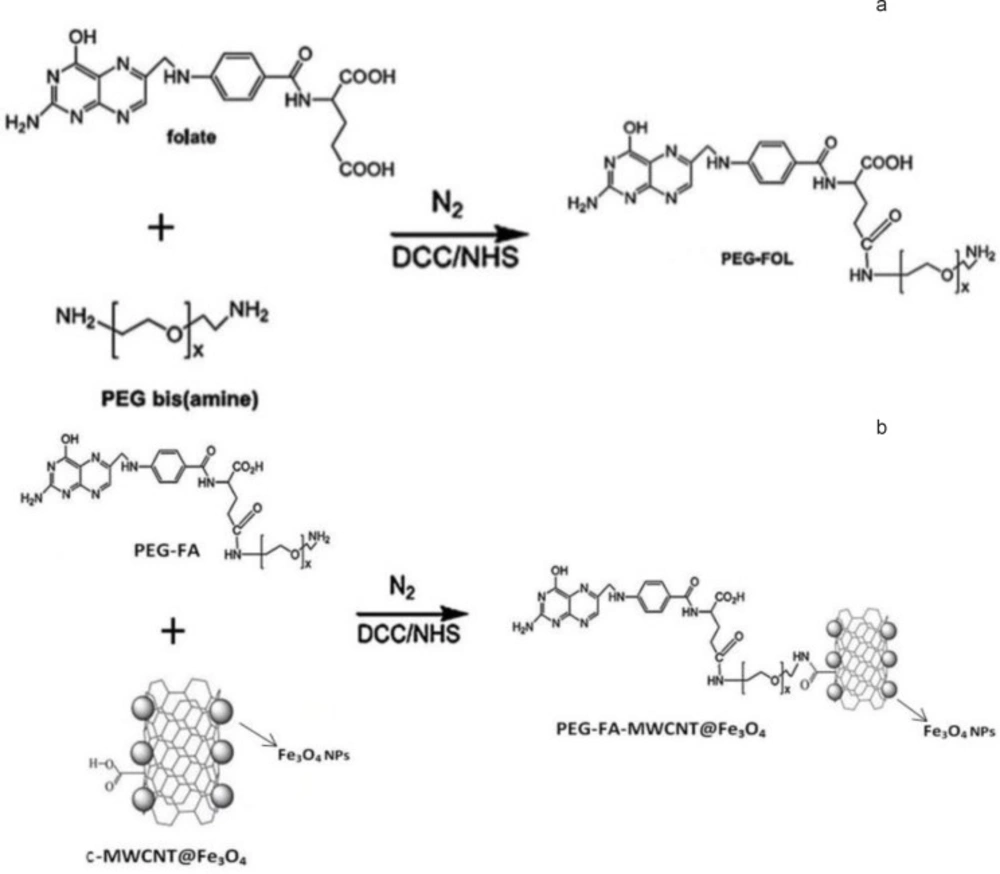
In order to activate folic acid carboxylic groups by NHS and DCC, 110 mg of folic acid was dissolved in DMSO (5 mL). After dissolving NHS (57 mg), DCC (100 mg), and PEG-bis-amine (50 mg) in triethylamine (50 mL), the mixture was stirred under nitrogen atmosphere at room temperature in dark for 24 h. Stoichiometric molar ratio of FA/DCC/NHS was 1:2:2. Unreacted triethylamine was removed by a rotary evaporator and the resulting product was diluted with 15 mL of deionized water and dialyzed against deionized water for several times (Spectra Por 6, MW cut off=1000) and then freeze-dried. Synthesis scheme of PEG-FA conjugate is shown in (Figure 1(a)) (5, 14).
Fabricating PEG-FA-SMWCNTs@Fe3O4
First, carboxylic acid groups of 70 mg c-SMWCNTs@Fe3O4 dissolved in 10 mL of phosphate buffer solution (50 mM, pH 6.0) were activated by 160 mg of DCC and 115 mg of NHS at room temperature in dark under nitrogen atmosphere for 6 h. PEG-FA (180 mg) was then added to the final mixture for coupling with c-SMWCNTs@Fe3O4 at room temperature in dark under nitrogen atmosphere for 20 h. Finally, the product was collected by magnetic separation, followed by three times of washing with deionized water and lyophilized. The synthetic scheme is described in Figure 1(b) (5, 10-12).
Results and discussion
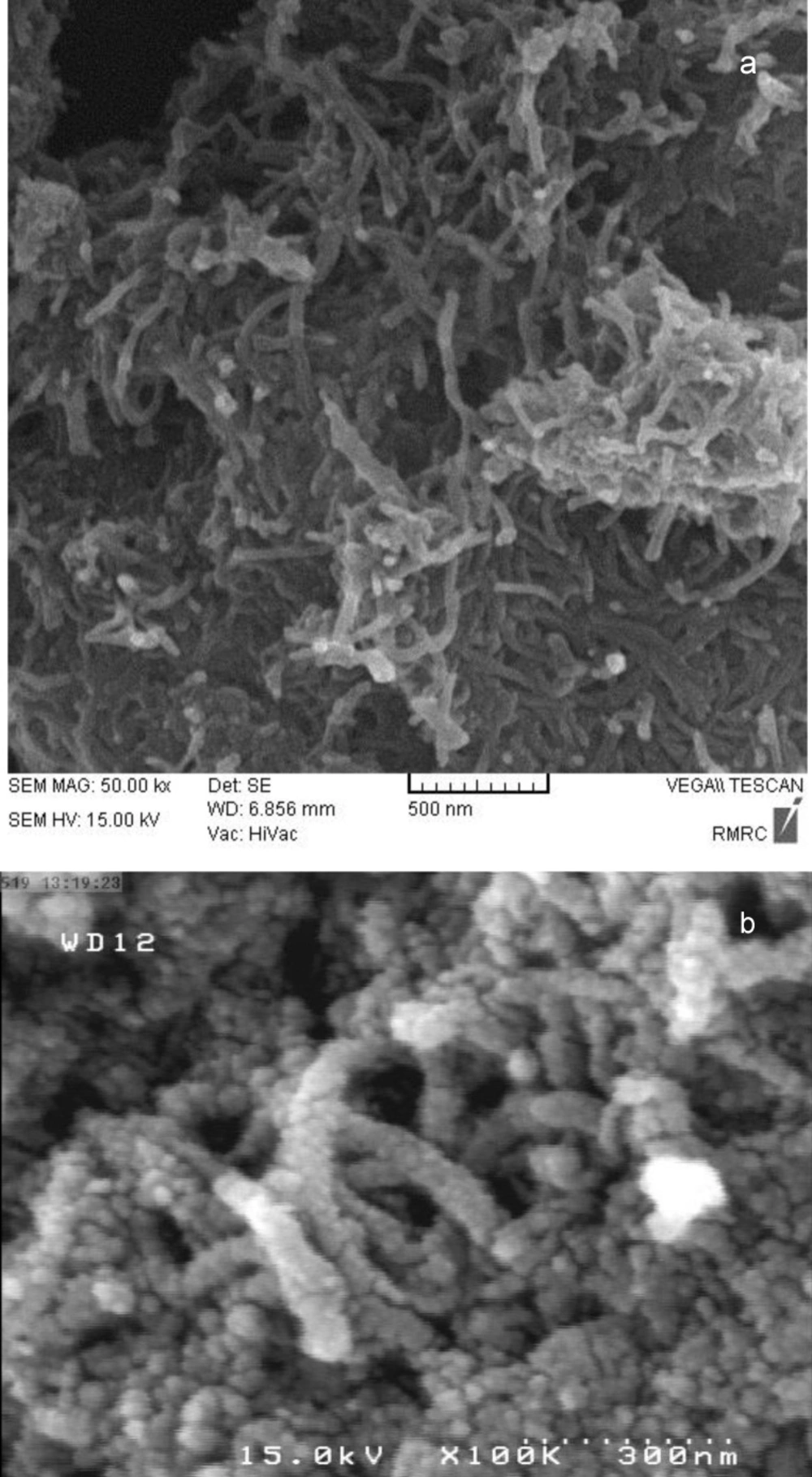
SEM images of c-SMWCNT and c-SMWCNT@Fe3O4 are shown in Figures 2(a) and 2(b). It is obvious that Fe3O4 nanoparticles successfully attached to the side walls of c-SMWCNT and c-SMWCNT@Fe3O4 was formed (Figure 2(b)).
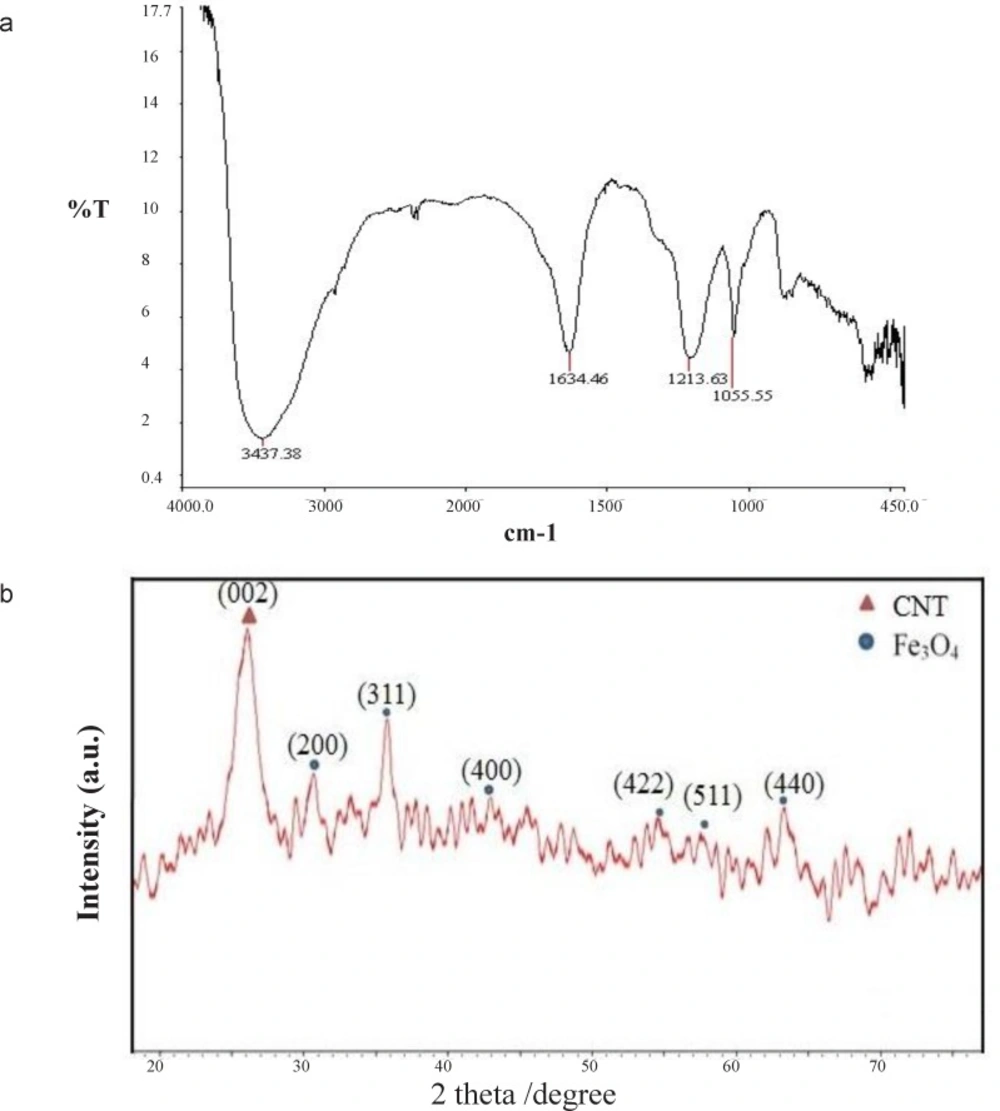
FTIR spectra of c-SMWCNTs are demonstrated in. Stretching C=O and C–O vibration at 1594.89 and 1213.63 cm-1 and strong stretching peaks of O–H at 3396.21 cm-1 indicated that carboxylic acid functional group was generated on the CNT surface(Figure 3(a)).
XRD pattern of decorated c-SMWCNTs with Fe3O4 NPs is illustrated in (Figure 3(b)). Diffraction peak at 2θ=25.99˚ was ascribed to the reflection of (200) plane of CNT, while the peaks at 2θ=30.17˚, 35.76˚, 42.87˚, 53.60˚, 57.52˚, and 63.35˚ were related to the (220), (311), (400), (422), (511), and (440) planes of the cubic Fe3O4 phase of inverse spinel structure (-).
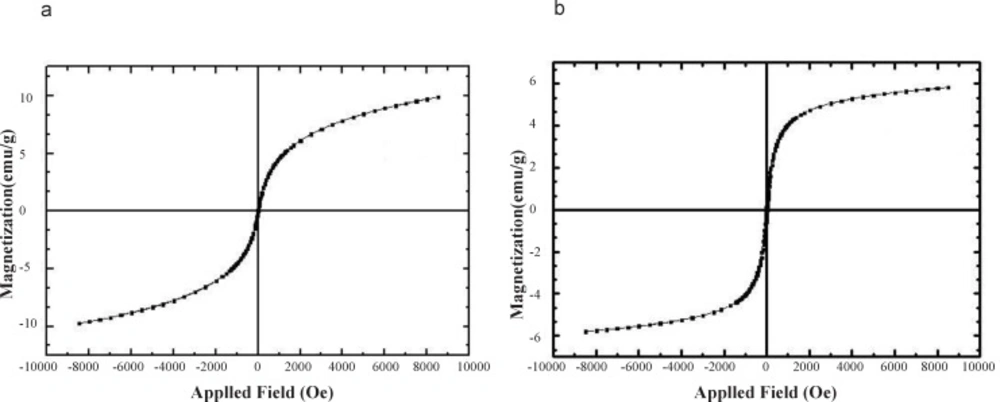
Typical room-temperature magnetization curves of magnetic Fe3O4 nanoparticles (a) and c-SMWCNT@Fe3O4 (b) are demonstrated in Figures 4(a) and 4(b). Saturation magnetization of the pure Fe3O4 and c-SMWCNT@Fe3O4 was 9.86 and 5.83 emu/g, respectively. It can be observed that the saturation magnetization of c-SMWCNT@Fe3O4 was comparatively less than that of pure Fe3O4 nanoparticles, which could be attributed to the presence of CNTs in c-SMWCNT@Fe3O4 sample. The curves exhibited zero remanence and coercivity, which proved that both Fe3O4 nanoparticles and c-SMWCNT@Fe3O4 had super-paramagnetic properties.
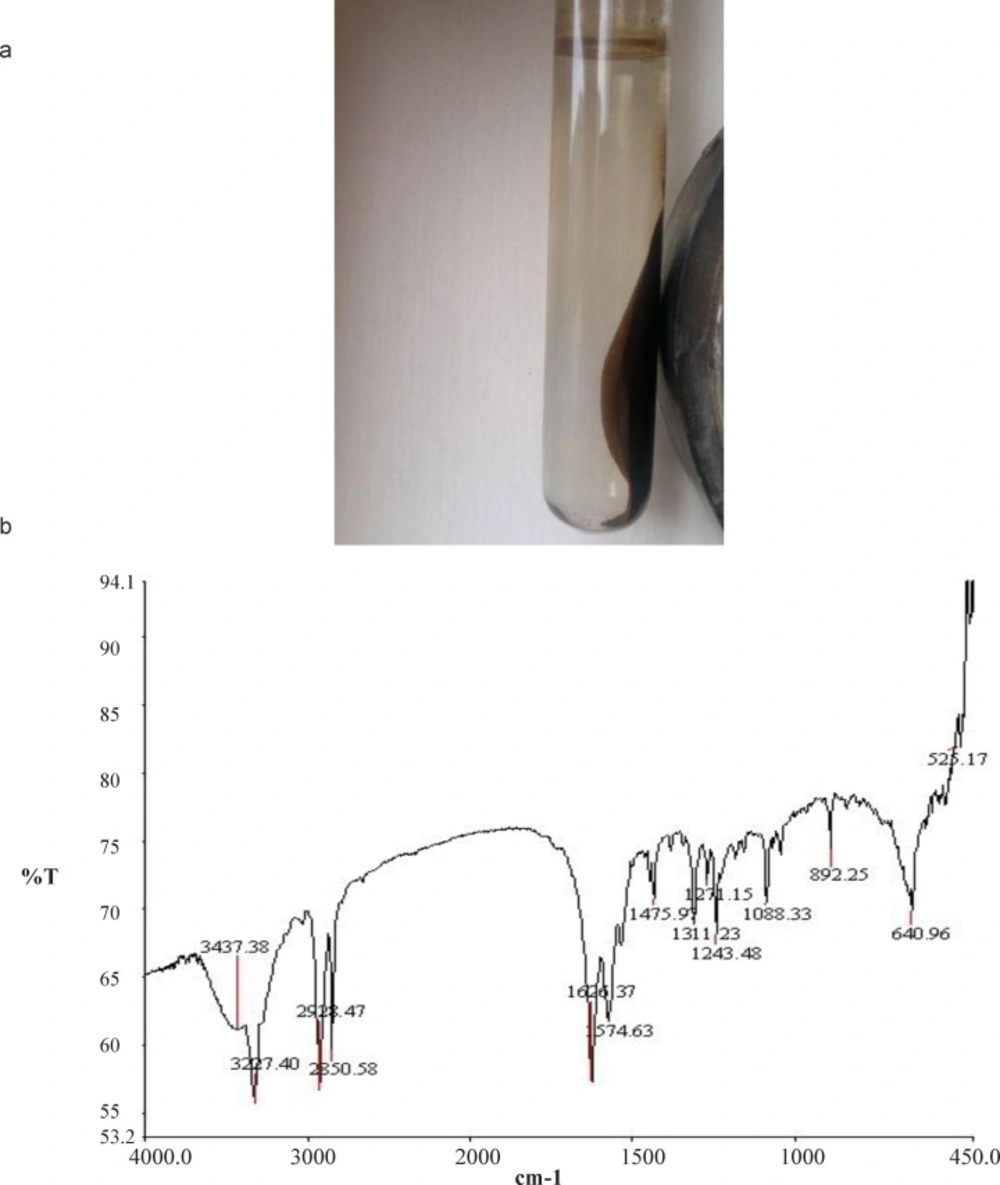
The band at 3437.38 cm−1 belonged to hydroxyl (O-H) stretching, while the absorption peak at1475.97cm−1 was attributed to the characteristic absorption band of phenyl ring. The bands at 2928.47 and 2850.58 cm−1 corresponded to C-H stretching vibrations, while the band at 1626.37 cm−1 belonged to C=O bond stretching vibration. Moreover, the bands at 1574.63 and 3227.40 cm−1 belonged to the bending and stretching modes of N-H vibration, respectively. The absorption peak at 1088.33cm−1 was related to the stretching vibration of the C-O-C vibration of PEG (20-22). Concurrence of Fe3O4 infrared absorption peaks at 525.17 and 640.96 cm−1 (Figure 5 b), which shifted to higher wave numbers, confirmed that Fe3O4 attached to PEG-FA-SMWCNT and PEG-FA-SMWCNT@Fe3O4 was formed.
Previous studies have approved the concept of forming NPs with a steric PEG barrier which could prevent their rapid uptake by mononuclear phagocyte system and promote their circulatory half-life (12, 16). More recent investigations have demonstrated that rapid RES uptake of NPs could be significantly reduced by changing their surface with poly (ethylene glycol) (PEG). PEG-modified NPs that are mostly prepared using a di-block copolymer of PLGA-b-PEG, liposome-PEG, dendrimer-PEG as an additive considerably prolong their half-life in the circulation owing to the presence of highly mobile and flexible PEG chains on the surface (16). Thus, the main reason for the attachment of PEG, FOL, and iron to carbon nanotubes could be preparing a novel nanocarrier with surface modification which could increase the potential for delivering anticancer drugs to the tumor site. This finding is in line with those of previous works (10, 12, 16, 23).
Conclusions
This study focused on functionalizing carboxylic acid groups on SMWCNTs (c-SMWCNTs), neutralizing the remainder acid of the solution by NaOH base to reach a pH value of up to 11, decorating c-SMWCNTs with magnetite (Fe3O4) nanoparticles (c-SMWCNTs@Fe3O4), and functionalizing c-SMWCNTs@Fe3O4 with PEG-Folic acid conjugated (PEG-FA- SMWCNTs@Fe3O4) . In accordance with obtained resultssuccess of the fully efficient, simple, and short-term method in attaching Fe3O4 to the surface of carbon nanotubes was confirmed using scanning electron microscopy, Fourier transform infrared spectroscopy, and X-ray diffraction spectroscopy. FTIR analysis also was used to investigate the link between PEG-FA and SMWCNTs@Fe3O4. This purposed nanocarrier has potential for future study in drug targeting.