Introduction
Linezolid (LZD), the oxazolidinone antibiotic, is widely used for treating the infections of gram-positive organisms that have resistance to some other antibiotics. In addition, LZD is applied for allogeneic hematopoietic stem cell transplantation (HSCT) settings widely (1). Anemia and thrombocytopenia induced by LZD hematological toxicities are common in patients (1-4), especially in those with long-term treatment of LZD. The most common cause of pure red cell aplasia (PRCA) was the mismatched ABO blood types between donors and recipients (5-7). However, reports concerning LZD-induced PRCA were rare (8-11). PRCA can lead to anemia, reticulocytes level decline (less than 1%) and various stages of erythroid lineages decrease, especially the absence of erythroid precursor cells (12). Patients who underwent allogeneic hematologic stem cell transplantation (allo-HSCT) were susceptible to microorganism infection such as the gram-positive organism infection. LZD was widely used for preventing microorganism infection for patients after HSCT.
Here, we reported a patient who underwent PRCA twice during long-term LZD treatment for central nervous system infection after allo-HSCT for MDS. We aimed to share some treatment experience and prevention strategies for analogous cases.
Case report
The patient and his parents had given the informed consent prior to the inclusion in the study. All human studies have been approved by Chinese Ethics Committee and performed in accordance with the ethical standards.
A 37-year-old man was diagnosed with myelodysplatic syndrome (MDS) in 2010. The primary symptoms for the patient were pale face (as the first symptom), anemia and thrombocytopenia. The bone marrow smear detected the dysplasia of three lineages presenting with obvious proliferation of 6.5% myeloblast and 7% promyelocyte. The chromosome analysis showed complex abnormalities: 46, XY, add (1) (p36), add (6) (p25), del (17) (q23) (11) /46, XY (3). Gene sequencing reveled that this patient presented with TKD (tyrosine kinase domain) mutation. The percentage of WT1/ABL and CBFB/MYH11 were 4.99% and 0.299%, respectively. MDS presented in this patient was classified to MDS-RAEB-I according to the classification of Word Health Organization (WHO) and intermediate-2 by International Prognostic Scoring System (IPSS). The patient had received allo-HSCT from a human leukocyte antigen (HLA)-matched unrelated donor for 4 months without any other treatment.
The patient and donor showed the identical ABO blood type. Before allo-HSCT, the preparative conditioning regimen consisted of Cyclophosphamide (Cy) 120 mg/kg and total body irradiation (TBI) 10 Gy. In the procedure of transplantation, the total dose of mono-nucleated cells (MNC) and CD34+ cells infused were 4.9×108/kg and 8.27×106/kg, respectively. The sustained engraftment of neutrophils and platelets was administrated at day 16.
Two years after HSCT, the patient suffered from refractory fever and headache for around 1 month. According to bio-chemical cerebrospinal fluid (CSF) test, he was diagnosed with central nervous system (CNS) infection without any pathogen detection. The empirical antifungal agent and antibiotics had little effect on the fever and headache of the patient, which included broad-spectrum drugs for gram-negative organisms and vancomycin (VCM) for gram-positive ones.
After changing drugs into LZD on October 18, 2012, the symptoms of fever and headache in the patient were gradually improved and the routine CSF test showed that CNS infection was relieved but the cerebrospinal fluid was still abnormal. However, the fever and headache symptoms reoccurred 1 week after stopping LZD treatment. According to the consultation from other related departments in our hospital, we considered it was probably CNS mycobcterium tuberculosis infection and then the anti-tuberculosis (TB) treatment with rifampicin, isoniazid, ethambutol and pyrazinamide was applied to suppress the return of fever and headache. Nevertheless, the patient with fever and headache continued to deteriorate after anti-TB therapy for 2 weeks, so the regimen was turned into LZD therapy on October 14th. Then the symptoms of fever and headache were improved again as well as the CSF test result.
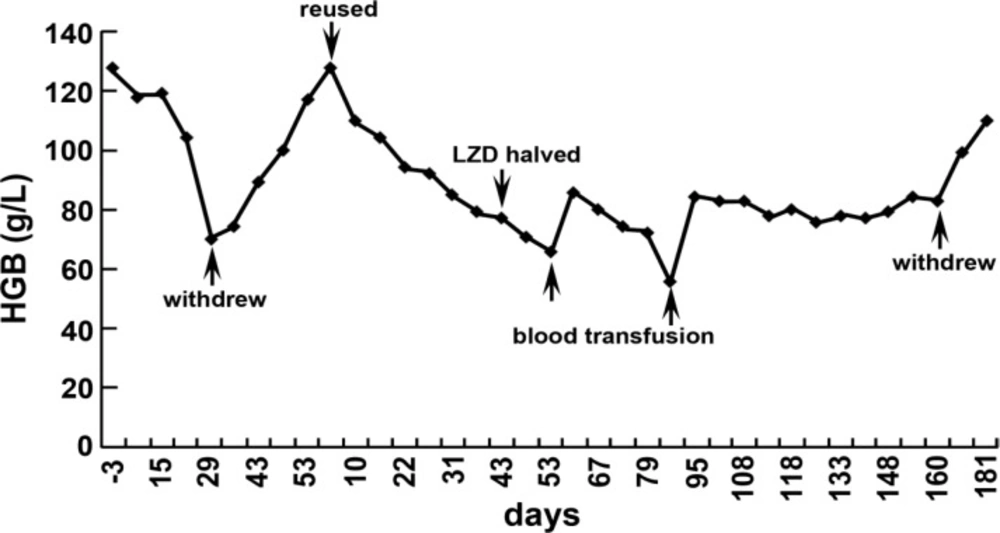
Complete blood count monitoring showed the hemoglobin was progressively declined from 128 g/L to 70 g/L since LZD was used for 28 days. The reticulocyte proportion was 0.23% and its absolute value was 0.005×1012/L. Bone marrow aspiration examination confirmed the presence of a hypo-proliferative anaemia with erythroid cells absence but without testing dysplasia or leukemia. The only concomitant medication was hepatoprotective drugs. Hemoglobin gradually increased to 117 g/L at day 24 after stopping LZD treatment. Thus, LZD was reused for treatment. The anemia relapsed 2 weeks later and the hemoglobin level was declined to 77 g/L after 6 weeks. At this time, the reticulocyte proportion was 0.21% and its absolute value was 0.005×1012/L. To remedy the anemia symptom, erythropoietin (EPO, 1wu, twice a week) was added and the dosage of LZD was halved. But there was no effect on anemia symptom and the anemia continued to deteriorate. After LZD was reused for 52 days, the hemoglobin concentration decreased to 66 g/L. The bone marrow smear examination was performed and the smear showed that there were marrow proliferation and obvious decrease of erythroid cells. But the dysplasia in cell lineages and leukemic cells was not observed.
The complete donor chimerism in the patient was confirmed by chimerism analysis. Then the patient accepted blood transfusion immediately and 1 month later, the hemoglobin level was only 55 g/L due to the progressive anemia. So, we conducted blood transfusion once again and the hemoglobin level rebounded to 82 g/L 10 days later. The halved dosage of initial LZD was continually administrated but the anemia symptom did not deteriorate anymore. LZD treatment was completely stopped after a full course of 5.5 months and the hemoglobin recovered to 110 g/L after 2 weeks (Figure 1). The proportion of reticulocyte was 1.65% (0.05×1012/L). The bone marrow examination showed a proliferative state and a decline of intermediate erythroblast.
Discussion
LZD is a new oxazolidinone antibiotic that can inhibit the synthesis of bacterial RNA and protein (13). Previous clinical researches indicated that long-term LZD treatment could result in diarrhea, headache, nausea and bone marrow suppression. The evidence about the close association of LZD and pure red cell aplasia (PRCA) was insufficient. To our knowledge, only 6 literatures documented about the LZD-induced PRCA (8-11), among which one case who suffered from MDS and PRCA within 1 month after allo-HSCT was reported (11). The case reported in the present study was different from that one in the suffering time (more than 2 years after HSCT) and the treatment course.
PRCA is a type of anemia with only red blood cell aplasia, which can be induced by thymoma, lymphoproliferative diseases, virus infections, autoimmune diseases and medication such as phenytoin sodium, EPO and chloramphenicol. All secondary PRCA may be related to the immune injury of abnormally proliferated T cells to eythroid (14-16). The patient in our study suffered from chronic liver graft-versus-host disease (GVHD), 1 year after HSCT. T cell was activated in the development of GVHD (17-19). The T cells proliferation can lead to immune impairing targeting erythroid (20, 21). In our case, EPO was used to cure the anemia at the second development of PRCA induced by LZD. But, EPO treatment showed little effect on LZD-induced anemia.
According to the manufacturer’s instruction, complete blood count should be carried out weekly for LZD therapy patients, especially for those with more than 2 weeks of LZD prevention, with concomitant drugs that can suppress bone marrow, with chronic infections or those with concomitant antibiotics (11, 22). Eric Senneville et al found that age > 58, alcoholism, concomitant diabetes and pre-existing anemia were the risk factors for LZD-induced PRCA. The age > 58 and pre-treatment hemoglobin < 105g/L were the independent risk factors for PRCA (23). In addition, Minson Q et al indicated that basic cardiovascular and urinary diseases, concomitant immunosuppressant therapy and baseline platelet count of 50-99×109/L were independent risk factors for 3-4 degree anemia induced by LZD (24). In our case, although the patient was relatively young and there was no cardiovascular or urinary disease, he had MDS history and underwent allo-HSCT before 2 years ago. Furthermore, he had taken azathioprine and thalidomide successively for more than 1 year for the chronic GVHD. All of these may be other potential risk factors contributing to the PRCA occurrence.
A new Italy research found that there was no obvious difference on the effect between the LZD dosage ≤ 600 mg and > 600 mg, but the side effects increased markedly in the > 600 mg dosage group (2). The two times of PRCA occurrence in our case was induced by LZD treatment at 600 mg dosage twice a day. At the second time, the dosage was halved, the CNS infection did not get worse and the anemia was slowly improved and finally got cured after LZD withdrew. It indicated that it was necessary to reduce the dose of LZD, when the side effects continued to worsen. The side effects would relieved but not disappear with LZD dose decrease.
In our case, the anemia occurred twice at day 22 and day 14 after LZD treatment. Eric Senneville et al (25) reported that the median time of anemia onset and LZD initiation was 7.4 weeks (4-16 weeks) for 45 patients which was more longer than our case. Reticulocyte was an important index that reflected the bone marrow erythroid function. Therefore, the reticulocyte might be declined earlier than peripheral anemia onset in PRCA patients. Correspondingly, routine monitor of reticulocyte for patients with long-term LZD therapy was recommended to predict the anemia occurrence. At present, no other effective methods could be taken to prevent or cure LZD-induced PRCA only when LZD was stopped. It was reported that oral Vitamin B6 could reverse LZD-induced PRCA (26), while no further investigation was taken to confirm it.
In summary, a case with PRCA twice after allo-HSCT was reported in this study. A long period LZD treatment was the primary factor resulting in PRCA. Anemia, myelosuppressants history may be other risk factors for PRCA onset. Abnormal T cell proliferation and the changes in the number of blood cells were common events in development of PRCA. Therefore, the complete blood count and reticulocyte count were necessary during LZD therapy. Besides, LZD dosage reduction and blood transfusion were effective for relieving LZD-induced PRCA. The patient with anemia would recover after LZD drug was withdrawn. However, a large number of studies should be conducted to verify our findings.
Conflict of interest
The authors have no conflict of interest to declare.