Introduction
Nanotechnology deals with the production and stabilization of various types of nanoparticles (1). Plants or natural resources have been found to be a good alternative method for nanoparticles synthesis, since this method does not use any toxic chemicals and does not require the use of high pressure, energy, and temperature. This green chemistry process has numerous benefits, including environmental friendliness, cost-effectiveness, and suitability for pharmaceutical and biomedical applications. At present, several groups of researchers concentrate on biomimetic approaches such as plant or plant leaf extracts, nuts, microorganisms and yeast to synthesize the metal nanoparticles called "green chemical or phytochemical" approach (2-5).
Antibacterial activity of the silver ion has been well established (6) and Ag is currently used to control bacterial growth in a variety of applications, including dental work, catheters, and burn wounds (7,8). In fact, Ag ions and Ag-based compounds are highly toxic to microorganisms, showing strong biocidal effects on as many as 12 species of bacteria including E. coli (9). Apart from these antimicrobial activities, Ag-NPs are also known to possess antifungal, anti-inflammatory, antiviral, anti-angiogenesis and antiplatelet properties (10, 11).
The betel (Piper betle) is the leaf belonging to the family Piperaceae. The P.betle leaves possess antifungal, antiseptic, antihelmintic, antihypertensive etc. medicinal properties (12). Besides, M. Mahfuzul Hoque et al., 2011 claim that ethanolic extract of P. betle leaves show antibacterial activity against some food borne pathogens (13).
The aim of this study was to synthesize AgNPs using aqueous leaves extract of P. betle, characterized the AgNPs and evaluated the antibacterial activity of Ag-Extract nanoparticles against some Gram positive and Gram negative bacteria.
Experimental
Collection of materials
Fresh young leaves of P. betle were collected from the local market of Noakhali, Bangladesh. Silver Nitrate (AgNO3), NaBH4 and Aniline were collected from the laboratory of Department of Pharmacy, Noakhali Science and Technology University (NSTU).
Preparation of P. betle leaf extract
About 30g of freshly, taxonomically authenticated healthy leaves of P. betle were collected in January 2014 from Noakhali district of Bangladesh. P. betle leaves extract was prepared according to K. Gopalakrishnan et al., 2012 (14). The leaves were washed thoroughly with double distilled water, cut into fine pieces and boiled with 150 mL double distilled water for 8-10 min. The extract was cooled to room temperature and filtered through Whatman filter paper. Finally, extract of leaves was stored at refrigerator at 4 ºc till further use.
Biosynthesis of silver nanoparticles
Ag nanoparticles were made according to the recipe described in the literature (Creighton, 1979; Suh JS et al.; 1983) (15, 16). Briefly, a 100-mL aqueous solution of 1.0 X 10–3 M silver nitrate was mixed with a 300-mL aqueous solution of 2.0 X 10-3 M sodium borohydride. Triply distilled water was used for solutions, and both solutions were chilled to ice temperature before mixing. By mixing both solutions, Ag ions were reduced and clustered together to form monodispersed nanoparticles as a transparent solution in aqueous medium. The Ag solution was turned yellow color during mixing. The solution was stirred repeatedly whenever some dark color appeared for approximately an hour until it became stabilized. At this point this solution of Ag nanoparticles was so stable that it did not change color for as long as several months without any stabilizing agent. Because the particle concentration of the solution is only 3.3 nM, it was concentrated 10 times using a rotary vacuum evaporator.
Preparation of Ag-Extracts Nanoparticles
These above two solutions were mixed continuously for 30 min by magnetic stirrer and then stood for 2 h. After that uncoated Ag-extract nanoparticles were prepared.
Coating of Ag-Extract Nanoparticles with Polyaniline
Coating on the Extract-AgNPs were done according to the K. Gopalakrishnan, 2012 (14). 2.7g aniline was dissolved into 300 mL deionized water. After that 68 mL (6 %) of H2O2 was added slowly at room temperature. This addition process was completed within 30 min. The reaction was carried out for 23 h at constant stirring rate. The final product was filtered, washed with distilled water and then dried at room temperature. Finally, samples were ready for further analysis (Antimicrobial activity).
Characterization Studies
The formations of silver nanoparticles were confirmed through visual assessment. The reaction mixture turned into dark brown color from its initial brownish-yellow color within 20 min of mixing. This color change indicated the formation of silver nanoparticles. The reduction of pure silver ions was monitored by measuring UV-Vis spectra of the reaction mixture. The absorption spectra of the samples were taken 240 to 540 nm using a UV–Vis spectrophotometer (Hitachi, Model U-2800 spectrophotometer). Water was used as the blank. Again, spectrum was recorded in FTIR (IR Prestige-21, Shimadzu) in the range of 4000–500 cm−1 at a resolution of 4 cm−1. TEM observations were carried out on an H-7100 electron microscope (Hitachi, Tokyo, Japan).
Test Microorganisms
Authentic pure cultures of Staphylococcus aureus ATCC 25923, Salmonella typhi ATCC 14028, Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were obtained from the Department of Microbiology, NSTU, Bangladesh.
Antimicrobial activity using Kirby-Bauer’s Disc diffusion method
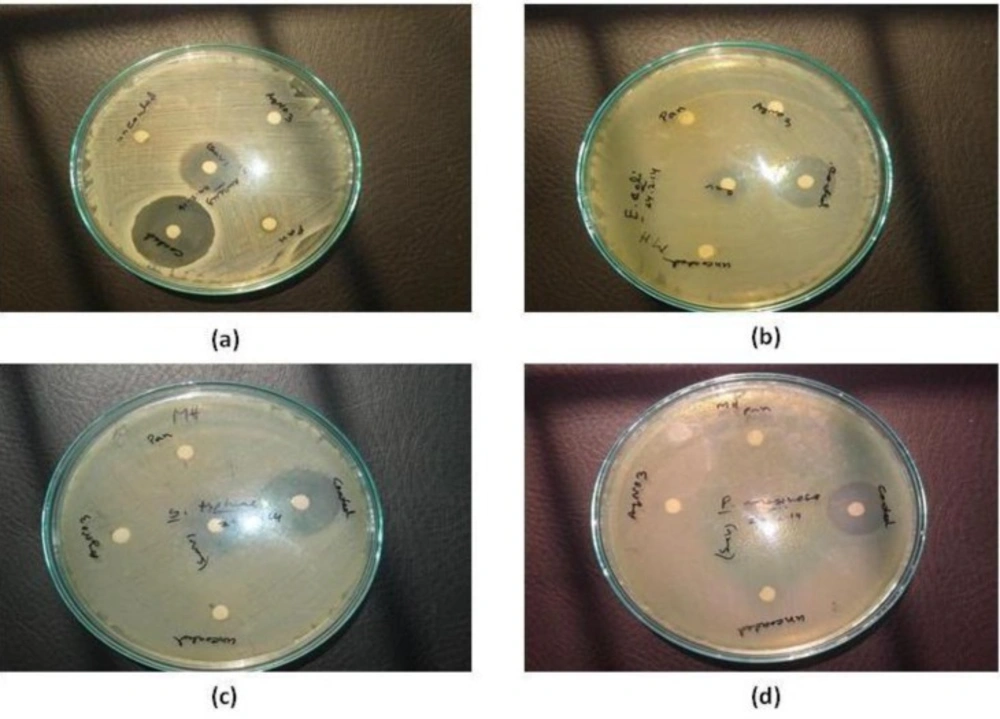
Antibacterial activities of the Ag-Extract nanoparticles were determined according to Kirby-Bauer’s Disc diffusion method (2006) with slight modifications. The Petri dishes were flooded with Mueller Hinton Agar and after solidification of agar 0.1 mL of diluted inoculums were spread over Mueller Hinton Agar in the dishes using sterile L spreader to achieve confluent growth of test organisms and allowed to dry for 10 min. The sterile readymade discs loaded with P. betle extract, AgNPs solution, uncoated AgNPs, coated Ag-Extract NPs. Then they were imposed on the inoculated plates. The plates were then incubated at 37 °C for 36 h. The plates were observed for the zone of inhibition. After incubation at 35 °C for 18 h, the different levels of zone of inhibition were measured. Zone of inhibition was measured using antibiotic zone scale. Norfloxacin was used as positive control.
Result and discussion
Characterization of the synthesized Ag Nanoparticles
Evaluation of Ag-NPs by visual assessment
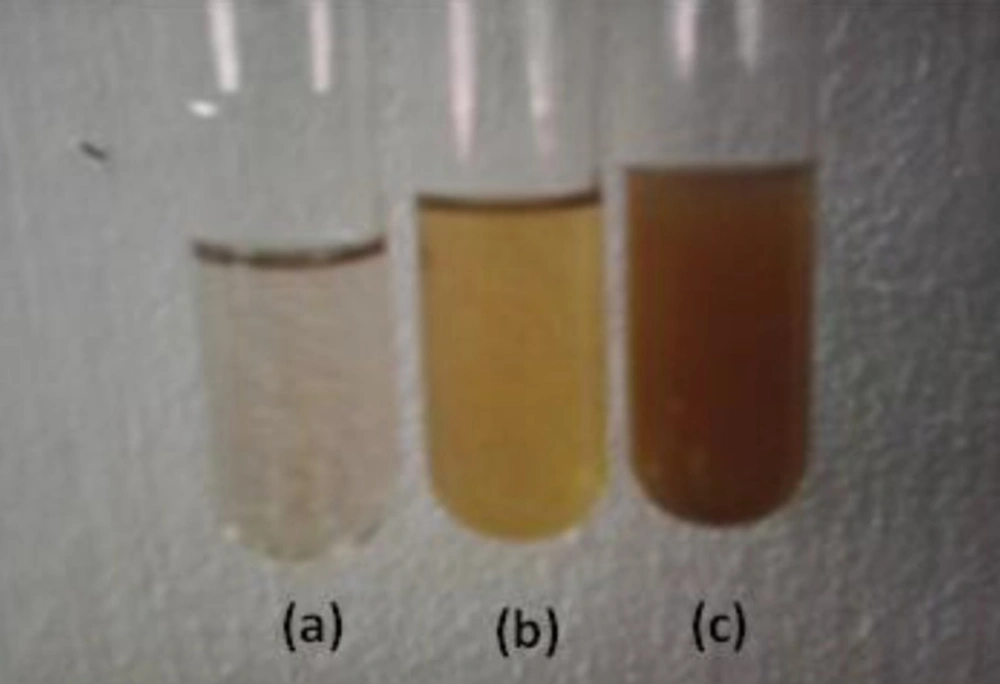
The formations of silver nanoparticles were confirmed through visual assessment. Within 20 min of the reaction, color of the mixture turned into dark brown from its initial brownish yellow color. This color changing indicated the synthesis of silver nanoparticles (Figure 1). The appearance of dark brown color may be due to the reduction of AgNO3 (17).
Evaluation of Ag-NPs by UV spectrum
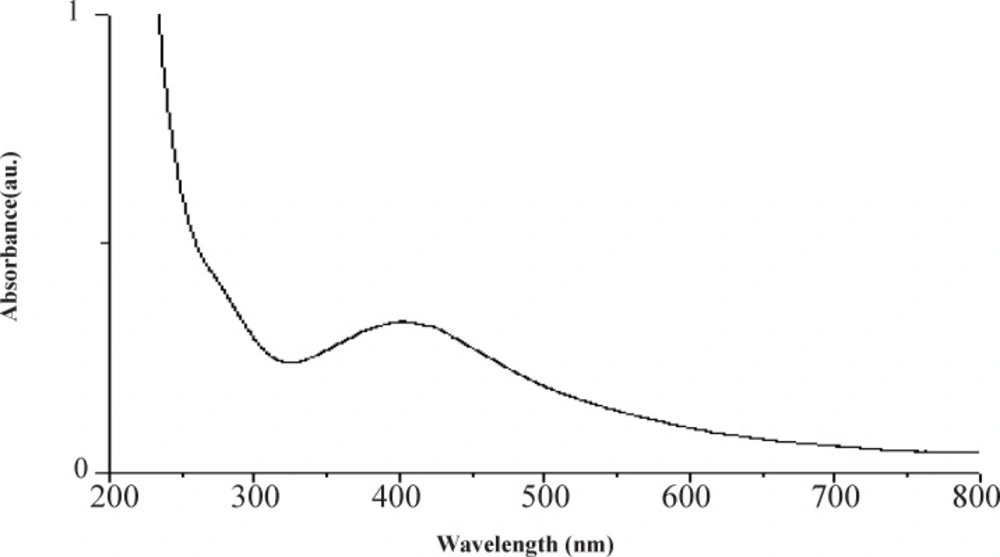
UV–Vis spectrum of reaction mixture at different wavelengths ranging from 300 to 700 nm showed strong absorption peak with centering at approx. 400 nm (Figure 2) indicated the formation of aqueous extract coated Ag-NPs.
Evaluation of Ag-NPs through FT-IR spectra
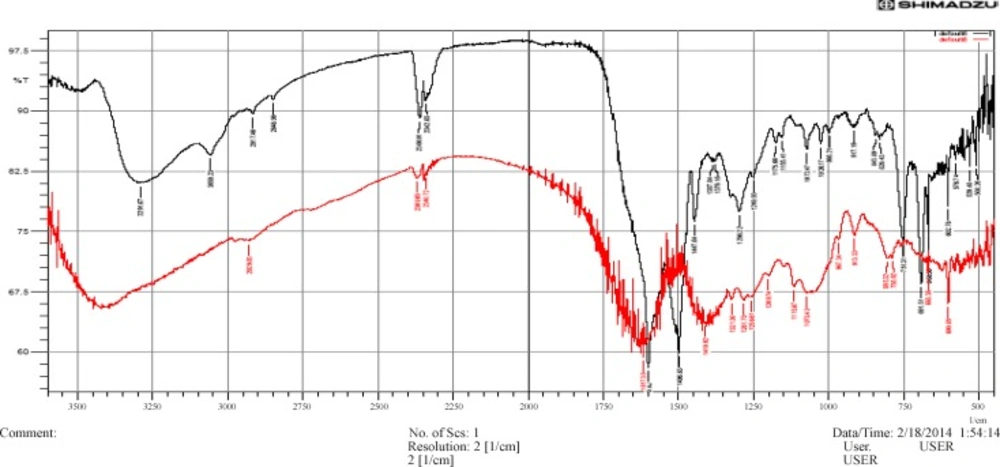
The FT-IR spectra were used to identify the possible biomolecules (including functional group) responsible for the reduction of the Ag+ ions and capping of the P. betle formed Ag-NPs. Figure 3, showed the FTIR spectra of P. betle aqueous extract and bio-synthesized Ag-NPs.
The possible functional groups of leaf extract involved in coating nanoparticle are identified by FTIR analysis. The intense absorption peaks at 3400 cm (curve-a, red color) and 3291.67 cm (curve-b, black color) correspond to N-H stretching of primary amine. The band observed at 3059.23 cm (curve-b) represents =C-H stretching. The weak band observed at 2917.46cm and 2848.98 cm (both in curve-b) indicates the H-C-H asymmetric and symmetric stretching of alkanes. The band observed at 2340.72cm and 2369.65cm (both in curve-a), which are nearly the same band in curve-b, denotes the presence of hydrogen bonded OH stretching of carboxylic acids in leaf extract which may be a reducing/coating agent for silver nanoparticles.
The band at 1617.38 cm (curve-a) and 1600.02 cm (curve-b) represents N-H bending vibration of primary amine. The peak occurs at 1496.83 cm (curve-b) for N-H bending vibration of sec. amine and at 1447.64cm (curve-b) C-H bending vibration of alkane (CH3). C-N stretch occurs at 1410.02cm. The band observed at 1387.84 cm and 1379.16 cm (both in curve-b) represent N=O stretching of nitro groups of leaf extract coated on nanoparticles. The arising of functional groups in FTIR spectrum indicates proper coating of leaf extract on silver nanoparticles. The bands at 1296.22, 1249.93, 1175.66, 1155.41 cm (curve-b), which are nearly the same in curve –a, denotes C-H stretching vibration of ester. Beside, C-O stretch occurs at 1072.47, 1026.17 (curve-b) where first is stronger and broader than the second. The band at 843.89, 829.43, 602.78, 668.36 cm shows C-H bending vibration of alkynes. The band at 751.31 cm and 691.51 cm represents the ortho substituted and mono substituted aromatic stretching respectively.
The FT-IR results imply that the Ag-NPs were successfully synthesized and capped with bio-compounds present in the P. betle extract by using a green method.
TEM analysis
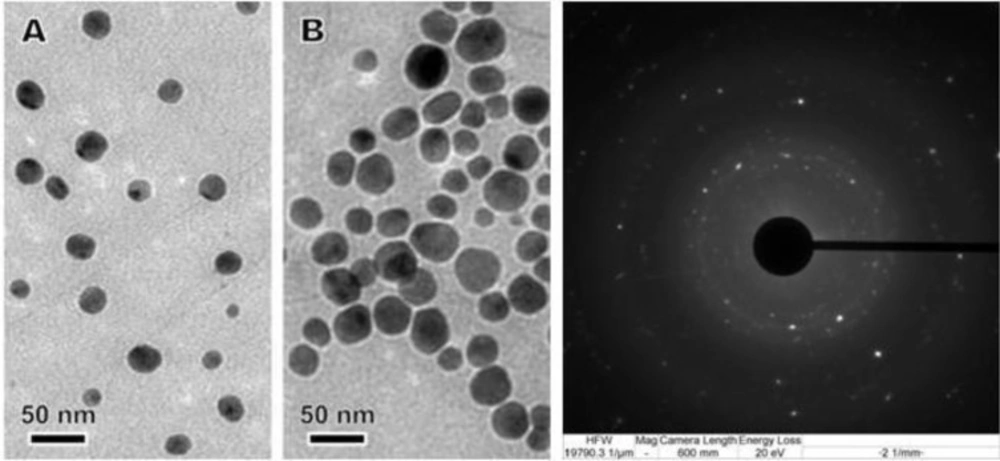
The TEM image in Figure 4 shows that Ag-NPs were well dispersed with a spherical structures and particle size ranging from 10 to 30 nm with some deviations. Of the total particles, approximately 20 % particles were of 10 nm, 50 % were of 20 nm and remaining 30 % particles were of 30 nm in size.
Assessment of antibacterial activities
| Items | Zone of inhibition (mm) | |||
|---|---|---|---|---|
| S. aureus | S. typhi | E. coli | P. aeruginosa | |
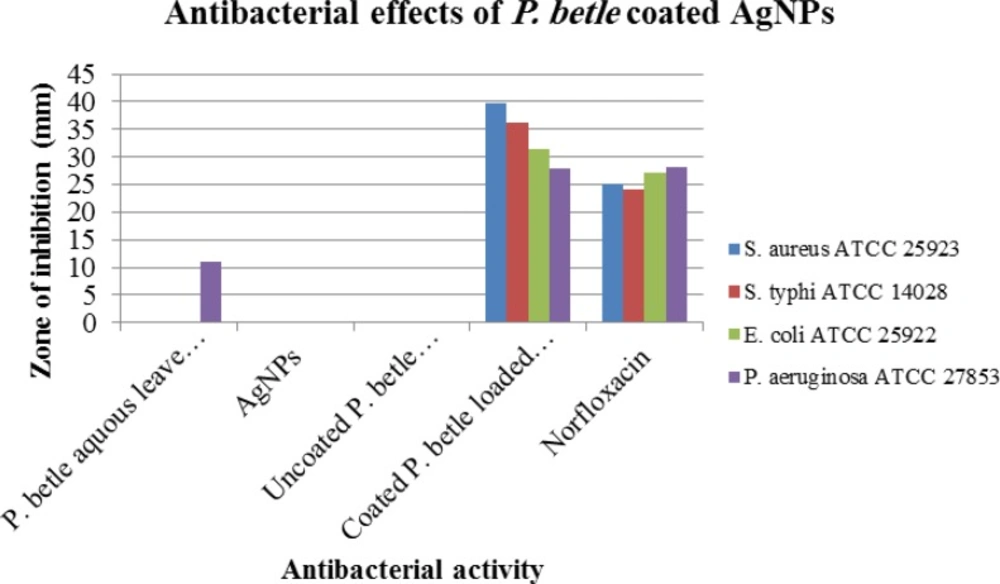
| P. betle aquous leave extract | - | - | - | 11.14±0.78 |
| AgNPs | - | - | - | - |
| Uncoated P. betle loaded AgNPs | - | - | - | - |
| Coated P. betle loaded AgNPs | 32.78±0.64 | 29.55±0.45 | 27.12±0.38 | 21.95±0.55 |
| Norfloxacin (control) | 22.15±0.40 | 21.40±0.40 | 19.23±0.40 | 17.62±0.40 |
Values are the mean of three replicates ± SD (standard error).
The antibacterial activity of the aqueous extracts of P. betle leaves were analyzed against both Gram-positive and Gram-negative bacteria, which are presented in (Figure 6, Table 1). After analyzing the above results, it is clear that gram positive bacteria S. aureus is more susceptible than other experimental species of gram negative bacteria - E. coli, S. typhi, P. aeruginosa. Maximum zone of inhibition 32.78±0.64 mm was found for S. aureus, whereas norfloxacin showed maximum 32.15±0.40 mm zone of inhibition for S. aureus. Again, maximum zone of inhibition 29.55±0.45 mm was found for S. typhi, 27.12±0.38 mm for E. coli and 21.95±0.45 mm for P. aeruginosa.
Conclusion
We have demonstrated a good method for developing a simple, safe, cost-effective, and ecofriendly preparation of AgNPs using aqueous extract of P. betle. The biosynthesized silver nanoparticles have spherical shapes and the particle size ranges from 10 to 30 nm approximately. The FTIR spectra revealed the involvement of hydroxyl moieties and N-H bonding in the formation of Ag-NPs. They showed potential antibacterial activity against both Gram-positive and Gram negative bacteria. In our study we also found, Gram positive bacteria are more susceptible on Ag-Extract NPs rather than Gram negative bacteria. Application of these synthesized AgNPs based on our findings may lead to valuable discoveries in various fields, including medical devices and in the pharmaceutical and biomedical industries. The results obtained by this study cannot be directly extrapolated to human; further studies should be undertaken to elucidate the exact mechanism of action by which AgNPs-extracts exert their antimicrobial effect which can be used in drug development program for safe health care services.