Introduction
Leishmaniasis represents a wide spectrum of clinical manifestations, ranging from a self-healing cutaneous lesion to fatal visceral disease. It is estimated that 350 million people are at risk of leishmaniasis, 12 million are affected and the annual incidence is about 2 million (1). In humans, cutaneous leishmaniasis (CL) caused by Leishmania major is self-limiting and affected patient is resistant to reinfection. This acquired immunity after natural infection or leishmanization suggests that an efficient vaccine is achievable. But there are still no available vaccines against human cutaneous leishmaniasis (2-4).
The main effect of particulate adjuvant/delivery systems is the effective delivery of antigen for enhanced uptake by antigen presenting cells (APCs), and dendritic cells (DCs) in particular. These adjuvants are most often taken up by classical APCs, such as DCs and macrophages, whereas B cells are not the prime target for these delivery systems (5). Various polymer- and lipid-based particulate antigen delivery systems have shown good potentials in potentiation of mucosal and systemic immune responses (6, 7). Sodium alginate, a natural polysaccharide polymer, can be easily cross-linked into a solid matrix with the addition of di- or trivalent cations (cross-linking in a water-in-oil emulsion results in the formation of microspheres). Alginate microspheres were used in several studies and showed good potential as vaccine delivery system and immunoadjuvant (8-10). Immunization of animals by alginate microspheres containing antigenic proteins elicited both humoral and cell-mediated immune responses (11).
The immunoadjuvant effect of Quillaja saponins (QS), extracted from Quillaja saponaria bark, is frequently reported (12, 13). One proposed mechanism for immunoadjuvant effects of saponins is by formation of mixed-micelles with cell proteins (14, 15). Controlling the cytokine production, clonal differentiation of complement cells in lymph nodes, enhancing the cytotoxic T lymphocytes (CTL) and natural killer cell activity, mitotic effect on lymphocytes and activation of macrophages and granulocytes have also been attributed to saponins (16, 17).
Quil A, a purified fraction of Quillaja saponaria extract, could intercalate into cell membranes through interaction with structurally similar cholesterol, forming ‘holes’ or pores (18). This may allow antigen to gain access to the endogenous pathway of antigen presentation and promote a CTL response, however it is currently unknown if the adjuvant effect of saponins is related to pore formation (19, 20).
One advantage of particulate delivery systems is their potential for co-delivery of antigen and adjuvant to the same APC. The immunogenicity of antigen and potency of an adjuvant could be substantially enhanced by their co-delivery in particulate delivery systems, like microspheres (21). At the present study alginate microspheres encapsulated with autoclaved Leishmania major (ALM) and Quillaja saponin adjuvant were studied for immunization against leishmaniasis.
Experimental
Materials, Animals, parasite and SLA
Sodium alginate (low viscosity grade) and purified Quillaja saponin were purchased from Sigma (St Louis, MO, USA). Autoclaved L. major (ALM) produced at Razi Vaccine and Serum Research Institute, Hesarak, Iran and used in clinical trials (3, 22-25).
Female BALB/c mice 6-8 weeks old were purchased from Pasteur Institute ?
The L. major strain (MRHO/IR/75/ER) used in this experiment is the same strain which has been used for preparation of experimental Leishmania vaccine and leishmanization (3, 22-27).
Soluble Leishmania Antigen (SLA) was prepared from promastigotes of L. major harvested at log phase (28), the protein concentration of SLA was determined using Lowry protein assay, and stored in small aliquots at -70C until use.
Preparation and characterization of Alginate microspheres encapsulated with ALM and QS
Methods of preparation and characterization of alginate microspheres used in this study has been reported in our previous publication (29). Briefly, sodium alginate solution (3.0% w/v, low viscosity) was dispersed in a n-octanol solution containing Span-85 (2.0% w/v) by sonication (Soniprep150, MSE, Sussex, UK). In the case of ALM and QS loaded microspheres, 3 mg of ALM and 20 µl QS (10 mg/ml in distilled water) were added to alginate solution. The W/O emulsion was rapidly added to a solution of calcium chloride in octanol (60 mL, 0.33% w/v) while stirring on a magnetic stirrer. After 10 min, 2 mL isopropyl alcohol was added dropwise to harden the formed microspheres. The microspheres were collected by filtration, washed with isopropyl alcohol and finally dried in a vacuum desiccator.
Immunization of BALB/c mice
Different groups of mice, 10 mice per group, were subcutaneously (SC) immunized (3 times at 3 weeks intervals) using one of the following formulations: 1- Alginate microspheres loaded with ALM and QS (ALM+QS)ALG (180 µg ALM + 10 µg QS/10 mg microsphere/100 µl PBS), 2- Alginate microspheres loaded with ALM and mixed with QS solution (ALM)ALG+QS (180 µg ALM/10 mg microsphere + 10 µg QS/100 µl PBS), 3- Alginate microspheres loaded with ALM (ALM)ALG (180 µg ALM /10 mg microsphere/100 µl PBS), 4- ALM solution mixed with QS solution, ALM+QS (180 µg ALM + 10 µg QS/100 µl PBS), 5- ALM solution, ALM (180 µg ALM/100 µl PBS), 6- PBS (100 µl).
Challenge with L. major
The immunized mice (7 per group) were challenged SC into left footpad with 1.5×106L. major promastigotes harvested at stationary phase (in 50 µl volume), at 3 weeks after the last booster and as a control right footpads were injected with the same volume of PBS. Lesion development was recorded in each mouse by measurement of footpad thickness using a metric caliper (Mitutoyo Measuring Instruments, Japan). Grading of lesion size was carried out by subtracting the thickness of the uninfected contralateral footpad from that of the infected one (30, 31).
In-vitro spleen cell response
Three mice from each group were sacrificed at week 3 after the last booster, at the same time as challenge experiment, the spleens were aseptically removed and a single-cell suspension was prepared by homogenization of the tissue, and the erythrocytes were disrupted using ammonium chloride. The splenocytes were washed and resuspended in complete medium (RPMI 1640-FCS) and seeded at 2×106/ml in 96-well flat-bottom plates (Nunc). The spleen cells were stimulated in vitro with SLA (10 µg/ml) or Con A (2.5 µg/ml), or medium alone and incubated at 37 oC in 5% CO2. Supernatants were collected at 72 h of culture and the concentration of IL-4 and IFN-γ were titrated using ELISA method according to the manufacturer’s instructions (32) (Bender Med Systems GmbH, Vienna, Austria).
Antibody isotype assay
Blood samples were collected from the mice before and at week 14 after challenge and the sera were used to titrate anti-Leishmania IgG total, IgG1 and IgG2a antibodies using ELISA method (Zymed Laboratories Inc., San Francisco, USA) according to the manufacturer’s instructions. Briefly, 96-well microtiter plates (Nunc) were coated with 50 µl of 10 µg/ml of SLA overnight at 4 oC. Plates were washed and blocked with 1% bovine serum albumin in Tween 20 (PBS-Tween). Serum samples were diluted to 1:200 with PBS–Tween 20 and applied to the plates. After incubation and washing, horseradish peroxidase (HRP)-conjugated antibodies were added. The amount of attached conjugated antibodies were determined by TMB substrate. H2SO4 2 M was used as stop solution. Optical density was determined at 450 nm using 630 nm as the reference wavelength (32).
Statistical Analysis
One-way ANOVA statistical test was used to assess the significance of the differences between various groups. In case of significant F-value multiple comparison Tukey test was used to compare the means of different treatment groups, P < 0.05 was considered to be statistically significant.
Results
Characterization of alginate microspheres
As reprted in our previous publication (29) the alginate microspheres used at the present study had a mean diameter of 1.92 ± 1.0 µm. The mean encapsulation efficiencies of ALM and QS in microspheres were 34.2 ± 6.7% and 31 ± 4.2%. ALM was released from microspheres with a burst release of 9.1 ± 3.0% after 30 min which was followed by a slow and continuous manner. After 7 days, a total of 35.7 ± 8.7% antigen release was occurred. The release of QS was started with a burst effect of 7.3 ± 1.9% after 30 min. After 7 days of incubation, cumulative release of 36.9 ± 4.7% was observed (29).
Lesion development after challenge
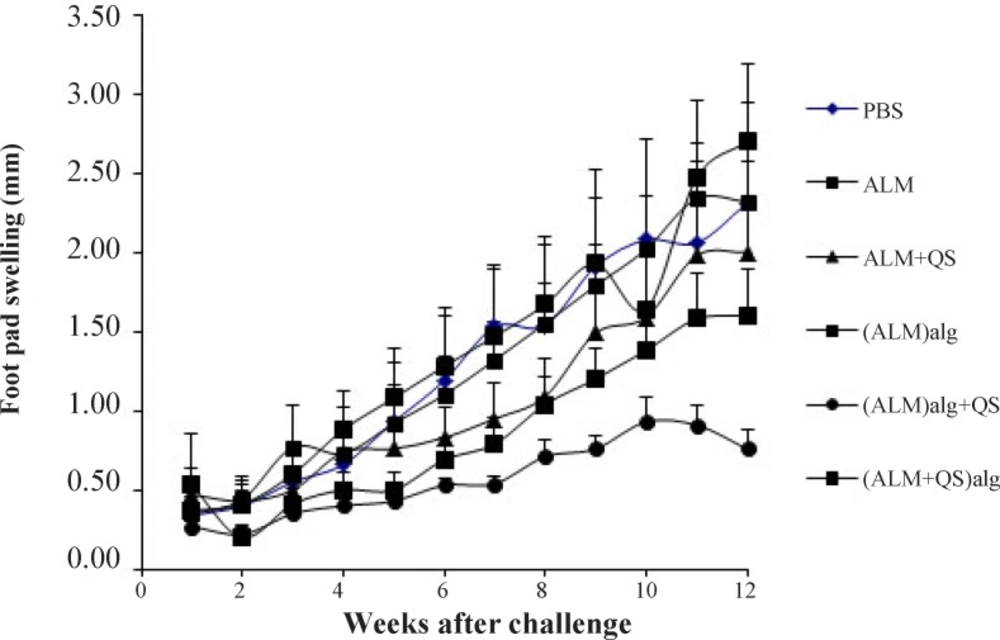
The protection against lesion development was monitored by weekly measurement of footpad thickness (Figure 1). The highest protection was seen in group of mice immunized with (ALM)ALG+QS (after 6th week, P<0.05 to P<0.0001). The intermediate protection induced by (ALM)ALG and ALM+QS was similar (P>0.05). The least protection was seen in groups immunized with PBS, ALM and (ALM+QS)ALG.
Footpad swelling in BALB/c mice immunized SC, three times in 3 weeks intervals, with (ALM+QS)ALG, (ALM)ALG+QS, (ALM)ALG, ALM+QS, ALM or PBS, after challenge with virulent L. major promastigotes. The mice were challenged in the left footpad with 1.5×106L. major promastigotes, 3 weeks after the last booster. The footpad thickness of mice was then measured on both footpads for 14 weeks. Each point represents the average increase in footpad thickness ± S.E.M. (n = 7).
In-vitro cytokine production by splenocytes
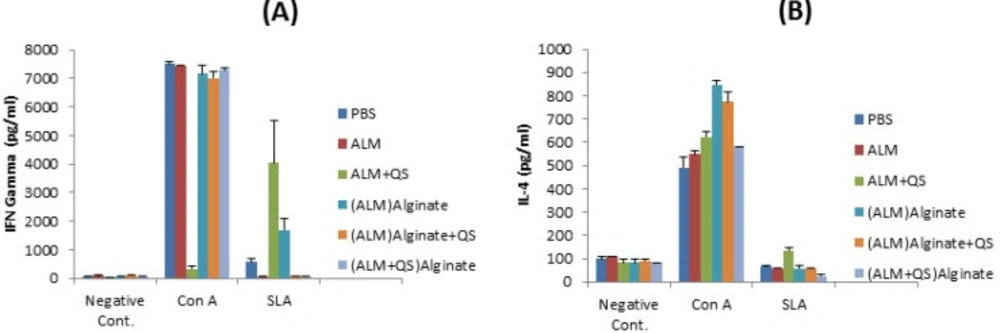
The supernatant of cultured splenocytes were used to titrate the level of IFN-γ and IL-4, cytokines indicative of Th1 and Th2 response, respectively. The significantly (P<0.01) highest levels of IFN-γ was seen in supernatant of splenocytes re-stimulated with SLA, in groups of mice immunized with ALM+QS and followed by (ALM)ALG (Figure 2A).
The significantly (P<0.01) highest level of IL-4 was seen in supernatant of splenocytes re-stimulated with SLA, in group of mice received ALM+QS. The IL-4 titers seen in other groups were nearly identical and lower than the negative controls (Figure 2B).
Splenic T-cell responses of BALB/c mice immunized SC, three times in 3 weeks intervals, with (ALM+QS)ALG, (ALM)ALG+QS, (ALM)ALG, ALM+QS, ALM or PBS. Twenty days after the last booster, their spleens were removed and the splenocytes were stimulated in vitro with either SLA (5 μg/ml) or SLA (10 μg/ml), concanavalin A (2.5 μg/ml), or with no stimulation. Production of IFN-γ (A) and IL-4 (B) were assessed on culture supernatants collected at 72 h of in vitro incubation using sandwich ELISA method. Cells from 3 mice per group were pooled. Each bar represents the mean and SEM of triplicate wells
Antibody response
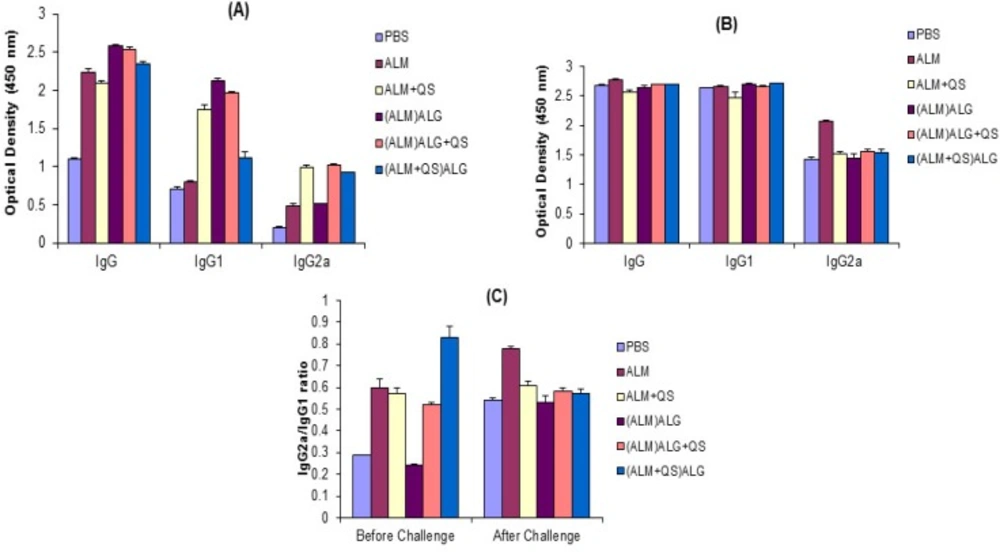
In order to determine the type of immune response generated in immunized mice, the anti-SLA specific IgG, IgG1 and IgG2a antibodies were titrated before (Figure 3A) and after (Figure 3B) challenge with L. major promastigotes. As shown in Figure 3A, the significantly (P<0.0001) highest titer of IgG2a (before challenge) was seen in sera of mice immunized with QS-containing formulations. The significantly (P<0.0001) highest IgG1 titer was seen in group of mice immunized with (ALM)ALG or (ALM)ALG+QS. The ratio of IgG2a/IgG1 in sera of mice immunized with (ALM+QS)ALG was significantly (P < 0.05) higher than the other groups (Figure 3C). Mice immunized with (ALM)ALG showed the significantly (P<0.0001) lowest IgG2a/IgG1 ratio.
Levels of anti-SLA-specific IgG, IgG2a, and IgG1 in sera of BALB/c mice immunized SC, three times in 3 weeks intervals, with (ALM+QS)ALG, (ALM)ALG+QS, (ALM)ALG, ALM+QS, ALM or PBS. Blood samples were collected from the mice, 3 weeks after the last booster (A) and 14 weeks after challenge (B). The SLA-specific IgG, IgG2a and IgG1 levels were assessed using ELISA method. Panel C indicates the ratio of IgG2a/IgG1 based on absorbance. The assays were performed in triplicate at 200-fold dilution for each serum sample. Values are the mean ± S.E.M
Challenge with L. major induced elevation of IgG, IgG1 and IgG2a antibodies in all the groups of mice compared with the antibody titers before challenge (Figure 3A). After challenge (Figure 3B), the significantly (P<0.0001) highest IgG2a titers were seen in group of mice received ALM. All other groups showed nearly identical IgG2a titers. The IgG1 titer in all groups of mice was similar (P>0.05). The significantly (P<0.001) highest IgG2a/IgG1 ratio was seen in mice immunized with ALM and followed by QS-containing formulations (Figure 3C).
Discussion
To enhance the immunogenicity of ALM, alginate microspheres encapsulated with ALM alone or ALM + QS were used to immunize mice. The protection rate, the extent and the type of immune response were evaluated in susceptible Balb/c mice.
QS as an immunomodulator adjuvant was co-administered with ALM, both mixed or co-encapsulated. The results of different tests were indicative of an intermediate potential of QS. The protection induced with ALM+QS was more than ALM group (Figure 1), but differences were not significant. ALM+QS induced higher IFN-γ and IL-4 than ALM group (P<0.001). Before challenge, ALM+QS group induced a higher IgG1, IgG2a titers, but IgG2a/IgG1 ratio compared to the group received ALM alone was not significantly different (P>0.05).
In phagocytosis of particulate antigen delivery systems, the mean diameter of particles is an important factor. Macrophages and dendritic cells could directly phagocyte the particles smaller than 10 µm in diameter (33). At the present study alginate microspheres with the mean diameter of 1.92 ± 1.0 µm which are readily phagocytosed by macrophages were prepared. Particulate antigens facilitate interaction with antigen presenting cells and induce stronger immune response compared to the soluble antigens (15, 34). As release profiles showed, within one week of release study, about 30% of antigen and adjuvant released from the microspheres. Therefore data generated might be attributed to both encapsulated and released antigen.
In murine model of leishmaniasis, recovery and protection depends upon generation of Th1 type of response, and progression of disease relates to the induction of Th2 type of response. The key cytokine and antibody subtype indicative for Th1 response are IFN-γ and IgG2a, while IL-4 and IgG1are key indicators of Th2 response (21, 35).
Alginate microspheres encapsulated with ALM induced a higher IgG1 and a lower IgG2a titers and a lower IgG2a/IgG1 ratio, as compared with free ALM. Antibody titers indicate that encapsulation of ALM in alginate microspheres skews the immune response toward a Th2 response. However, the level of IFN-γ in group of mice immunized with (ALM)ALG was significantly (P<0.01) higher than the group received ALM alone, which is contrary to the antibody titers. After challenge with L. major promastigotes the lesion size was significantly smaller in group of mice received in (ALM)ALG than the group received ALM alone (P<0.05, after 4 weeks), therefore, the microspheric formulations showed to induce a higher protection. Thus while the antibody titers are against the microspheric formulation, the cytokine titers and the lesion size showed a positive potential for microspheres in induction of protection in murine model of leishmaniasis, compared with animals immunized with ALM alone.
In our previous studies in rabbit, tetanus toxoid (TT) as a model antigen was encapsulated with alginate microspheres and administered nasally. The IgG, sIgA, and antitoxin titers induced with (TT)ALG were significantly (P<0.01) higher than the TT solution.
The Alginate microspheres were also mixed with QS solution. The protection induced by (ALM)ALG+QS was significantly higher than (ALM)ALG (after 6th week, P<0.05 to P<0.0001).The (ALM)ALG+QS could induce higher IgG2a/IgG1 ratio and lower IFN-γ levels, compared with (ALM)ALG group (P<0.0001).
Co-encapsulation of QS with ALM in the microspheres ((ALM+QS)ALG) had a negative effect on protection. In mice immunized with (ALM+QS)ALG, the IgG2a/IgG1 ratio was higher (P<0.0001) than (ALM)ALG+QS, however, the lesion size was more than (ALM)ALG+QS group (P<0.0001).
Several authors have shown that Quillaja saponins (including QS-21) stimulated the production of CTLs and induces Th1 cytokines (IL-2 and IFN-γ) and antibodies of the IgG2a isotype to protein antigens (36, 37). The potential of Quil A in induction of Th1 immune responses against murine visceral leishmaniasis was demonstrated in their combination with the fucose–mannose ligand of Leishmania donovani (FML). It was shown that the QS-21/FML and Quil A/FML groups achieved the highest IgG2a response, while Quil A/FML developed the strongest delayed type of hypersensitivity (DTH) and QS-21/FML animals showed the highest serum IFN-γ concentrations among five kinds of adjuvants (38). Quil A or QS-21 could also elicit antigen-specific Th1 responses against Plasmodium falciparum, foot-and mouth disease and measles virus in mice (38).
In the other hand, these are several studies in which a mixed Th1 and Th2 immune responses have been reported for saponin adjuvants. Saponins have been found to enhance phagocytosis and stimulate secretion of cytokine such as IL-1, IL-2, IL-4, IL-6, IL-10, IFN-γ and TNF-. Such broad range of cytokines is consistent with the mixed Th1/Th2 responses (39). Xie et al. were used from Platycodon grandiflorum saponin (PGS) as adjuvant for ovalbumin. After S.C. injection in mice, PGS showed a balanced Th1 and Th2 immunological response (40). For the immunotherapy of dogs, when Quil A was added to leishmune vaccine, average of the clinical scores was decreased, but average of CD4+ Leishmania-specific lymphocytes was increased (41). P. notoginseng saponin elicited a Th1 and Th2 immune response in mice by regulating production and gene expression of Th1 and Th2 cytokines (42).
The type of immune response that is reported after immunization of saponin-adjuvanted vaccines (Th1 or mixed Th1/Th2) depends not only on the adjuvant itself, but also on the factors like the antigen, administration route and immunization schedule (39).
The other explanation for different immune responses is related to lipophilic acyl side chain of saponins which is shown to be responsible for the remarkable stimuli for CTL production against exogenous proteins and instability under physiological conditions (43). The spontaneous deacylation of saponins in aqueous solution (44), could lead to production of the deacylated saponins. These deacetylated saponins are significantly less toxic and elicit Th2 responses while fail to stimulate either a lymphoproliferative response or the formation of CTL (43).
In summary the results of this study showed that QS adjuvant induced a mixed Th1/Th2 immune response.