Introduction
Alopecia is a common disorder which results in diminution of visible hair. Alopecia is not a life-threatening disorder, yet it has certain impacts on social life, on mental health, and on the life quality of patients (1, 2). Currently, dermatologists have developed several medical treatments for hair loss or thinning. Despite the new technologies and therapies, many people still suffer from hair loss, thinning and baldness (1, 3 and 4). Therefore, it is important to develop new therapies to stop hair loss and to enhance hair growth. In this regard, many people resort to alternative or traditional medicinal remedies.
The importance of traditional medicine is increasing day by day although the prevalence varies depending on the ethnological, medical and historical background of every country. People return to nature and traditional medicine and several governments support the use of medicinal plants, which are the oldest known health-care products. A large proportion of the population in a number of developing countries still relies on traditional medicine and local medicinal plants to satisfy their primary healthcare needs. According to the World Health Organization (WHO), not only the direct use of plant constituents as therapeutic agents, but also utilization of these plants as basic materials for the synthesis of drugs or as models for pharmacologically active compounds is important for pharmacological research and drug development (5, 6). The need for in-vitro/in-vivo research on the medicinal plant species is predominant to fill the gaps between the traditional uses and in-vitro studies, pharmacological studies, toxicity profiling and clinical trials (7). One of the medicinal plants is Delphinium staphisagria. Delphinium is a genus of about 300 species in the family of Ranunculaceae (8, 9). Especially, in Mediterranean tradition medicine, seeds of Delphinium species are being used for enhancement of hair and treatment of hair loss since ancient times. The seeds of many Delphinium species (D. ajacis, D. brunonianum, D. caeruleum, D. consolida and D. staphisagria) have long been used to produce insecticide in western medicine. In Asia and Europe especially D. staphisagria have been used in the treatment of scabby, for killing head louse (antiparasitic), the treatment of hydrops, as purgative, antiodontalgic, aphrodisiac and emetic in case of poisoning (10, 11). Marin et al. (2011) studied in-vitro and in-vivo trypanocidal activity of flavonoids from D. staphisagria against Chagas disease which is caused by a parasite (12). Ramirez-Macias et al. (2012), demonstrated that leishmanacidal activity of several flavonoids from D. staphisagria (13). Two products with D. staphisagria extract as active component are commercially available - in Germany, a painkiller for soft-tissue injuries and in Turkey, a hair care lotion (http://www.tuanadogalyasam.com/ENTELE-OTU-LOSYONU,PR-121.htlm). .D.staphisagria is called “entele”, “bit otu”, “papaz out”, “hazeran” or “mevzek” in Turkey (http://www.dogaltedavi.net/f92/mevzek-otu-stephanskraut-delphinium-staphisagria-4556.html).
Although keratinocytes are primary constituents of the hair follicle, there are approximately 20 different cell types in the hair follicle, one of which is endothelial cells (14, 15). The growth process of hair includes four different periods: growth (anagen), regression (catagen), rest (telogen), and shedding (exogen) (1, 3, 16 and 17). During the anagen phase, significant vascularization occurs surrounding the hair follicle and during the catagen and telogen phases degeneration and disappearance of blood vessels are observed. Thus, active hair growth is accompanied by the induction of angiogenesis to meet the increased need for blood flow, other factors and nutrition, which are necessary for the rapid proliferation of follicular keratinocytes and the subsequent elongation and thickening of the hair shaft. Enhanced vascularization of the hair follicle has been shown to promote hair growth and increase the diameter of hair follicles and hair shafts (18, 19 and 20). Different studies demonstrated that anagen is associated with angiogenesis. These observations suggest that angiogenesis is a quite prominent step for hair follicle recovery.
Interestingly, despite the special effects of the seeds of D. staphisagria on promoting hair growth, --to our knowledge-- we could not encountered any reports in the literature explaining the mechanism of such an activity.
Therefore, we decided to determine any possible cytotoxic effect that D. staphisagria seeds might exert on human keratinocytes (HaCaT) and human endothelial cells (HUVECs), and in-vitro angiogenic capacity of these seeds on the HUVECs, which constitute the main components of hair follicle. Based on our results, we propose that D. staphisagria seeds enhance hair growth by increasing the proliferative activity of the hair follicles through elevated angiogenesis.
Experimental
Cell culture
HaCaT (immortalized human keratinocyte) cell lines were obtained from the DSMZ (German Collection of Microorganisims and Cell Cultures). HaCaT cells were maintained as a monolayer in DMEM High Glucose (Sigma) containing 10 % (v/v) Foetal Bovine Serum (Sigma), Penicillin-Streptomycin (Sigma) and sodium hydrogen carbonate.
Human Umbilical Vein Endothelial Cells (HUVECs) were purchased from the ATCC (American Type Cell Collection). Cells were incubated and grown in Nutrient Mixture F12 HAM (Sigma) medium supplemented with 20 % heat-inactivated fetal calf serum (Sigma), heparin (0.1 mg/mL), endothelial cell growth supplement (ECGS, 0.05 mg/mL) (Sigma), Penicillin-Streptomycin (Sigma) and sodium hydrogen carbonate. HUVEC and HaCaT cells were cultured in an incubator with a humidified atmosphere (70%) at 37 ºC, and 5% CO2.
Preparation of Delphinium staphisagria extract
Seeds of Delphinium staphisagria were bought commercially from a local herbalist in Eskisehir/Turkey. Intact seeds were prepared as traditionally prescribed by A. Tansu Koparal. Firstly 2.5 g of the seeds was boiled in 250 mL apple commercial vinegar (Kühne) for 20 min. The solution was incubated overnight at room temperature (http://www.sifamarket.com/mucize-bitkiler/entele-otu-faydaları.html). The remaining liquid was sterile-filtered (0.45 μm filter) to approximately 100 mL. Several concentrations were prepared with fresh medium. Also, we prepared water extracts with using the same procedure.
Cytotoxicity assay
The effects of Extract which is prepared from Seed of Delphinium staphisagria (ESDS) (vinegar and water) were determined by using in-vitro colorimetric MTT [3-(4,5-dimethythiazol-2-yl)-2,5-diphenyltetrazolium bromide] cytotoxicity assay (21). The cytotoxic activity was determined after 24 and 48 h. All experiments were repeated at least three times.
HUVECs (8 x 103 cells/well) and HaCaT cells (2 x 104 cells/well) were seeded into 96-well microtiter tissue culture plates (Techno Plastic Products AG) with a final volume of 100 μl. After 24 h of attachment for HUVEC and 48 h of attachment for HACAT, the culture medium was removed and the cells were treated with different concentrations (1-300 µg/mL) of the test samples. MTT assay was performed as previously described (22). For references, old medium was replaced with fresh medium without the dye. In order to test cytotoxicity, the ESDS of each concentration were cultured with HUVEC and HaCaT cells for 24 h and 48 h, respectively. During this period after each day, the medium was aspirated and 100 µL fresh medium containing 0.5 mg/mL MTT (Sigma) dissolved in Phosphate Buffer Saline (PBS) was added to culture wells. The plates with added MTT solution were then wrapped in aluminum foil and replaced in the 5 % CO2 incubator for 2 h. At the end of this period, the medium was removed and the formazan crystals formed by MTT metabolism were dissolved by addition of 100 µL DMSO to each well. The plates were gently mixed on a plate shaker approximately for 5 min, and their absorbance values were read at 570 nm with a microtiter plate reader (Bio-Tek, ELX808IU, USA). Every test concentration had the eight replicates per assay and every experiment was carried out on at least three separate occasions. (+)-Thalidomide is used as positive control for HUVECs as several studies suggested thalidomide to inhibit angiogenesis (23, 24). The SPSS (Statistical Package for Social Sciences) software has been used for the statistical analyses of assessment of the MTT assay. Data were evaluated using one-way ANOVA (Analysis of Variance) followed by the Tukey’s test. A value of p<0.05 was considered significant.
Matrigel tube formation assay
The assay was performed as previously described (25, 26). For this assay HUVECs were serum-deprived by culturing in endothelial cell basal medium-2 (EBM-2; Cambrex Bio Sciences) with 2% FBS for 4 h. The serum-deprived cells were plated at the density of 4x104 cells/well on Matrigel (BD- Biosscience), which coated the wells of 96-well plates and had been equilibrated with EBM-2 medium containing test samples. After 12 h, the endothelial cell-derived tube-like structures were visualized under an inverted microscope (Olympus IX71) and photographed (Olympus DP71 camera) at a magnification of 10x.
Results
Effect of the Delphinium staphisagria seeds on the viability of HUVEC and HaCaT cells
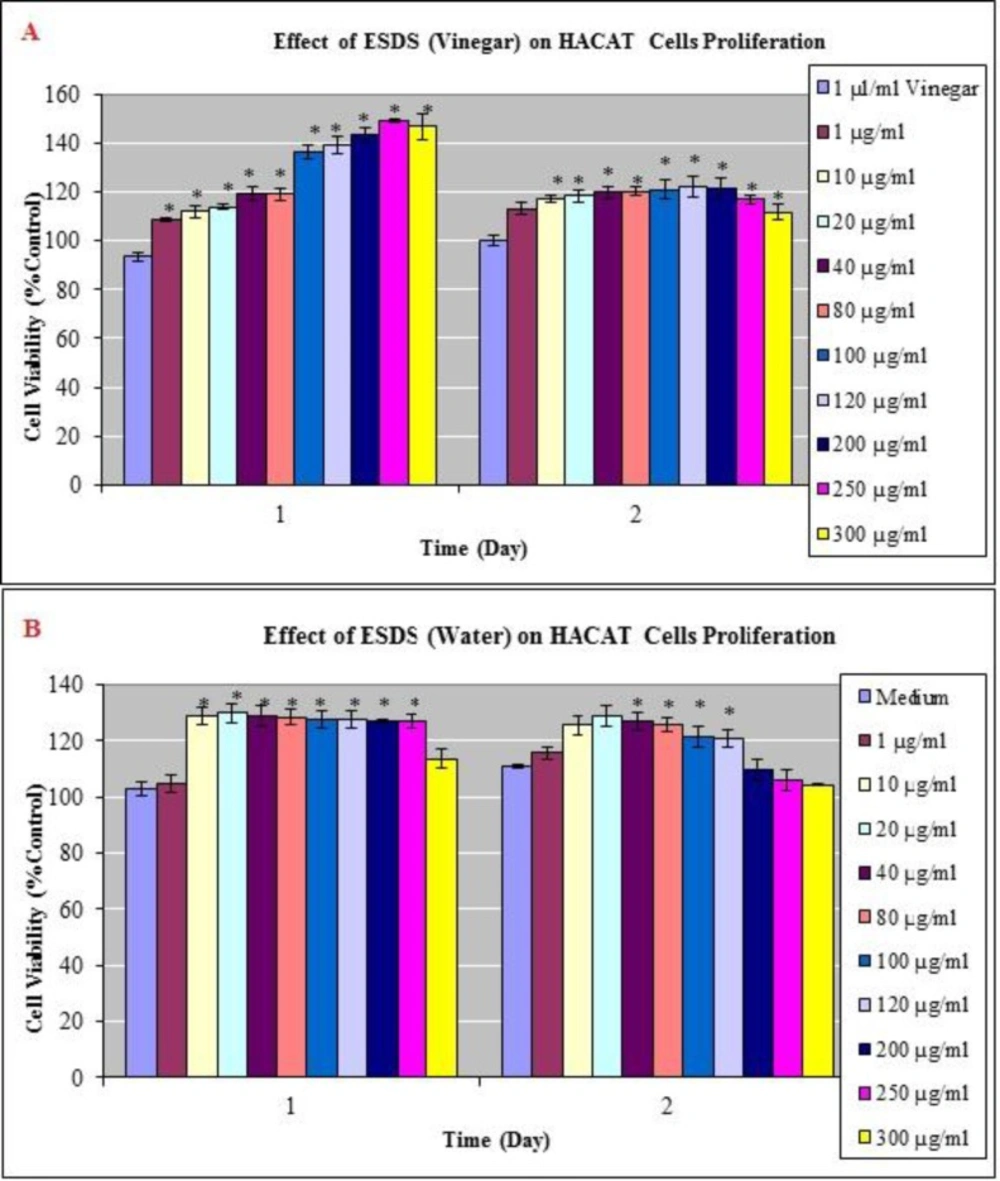
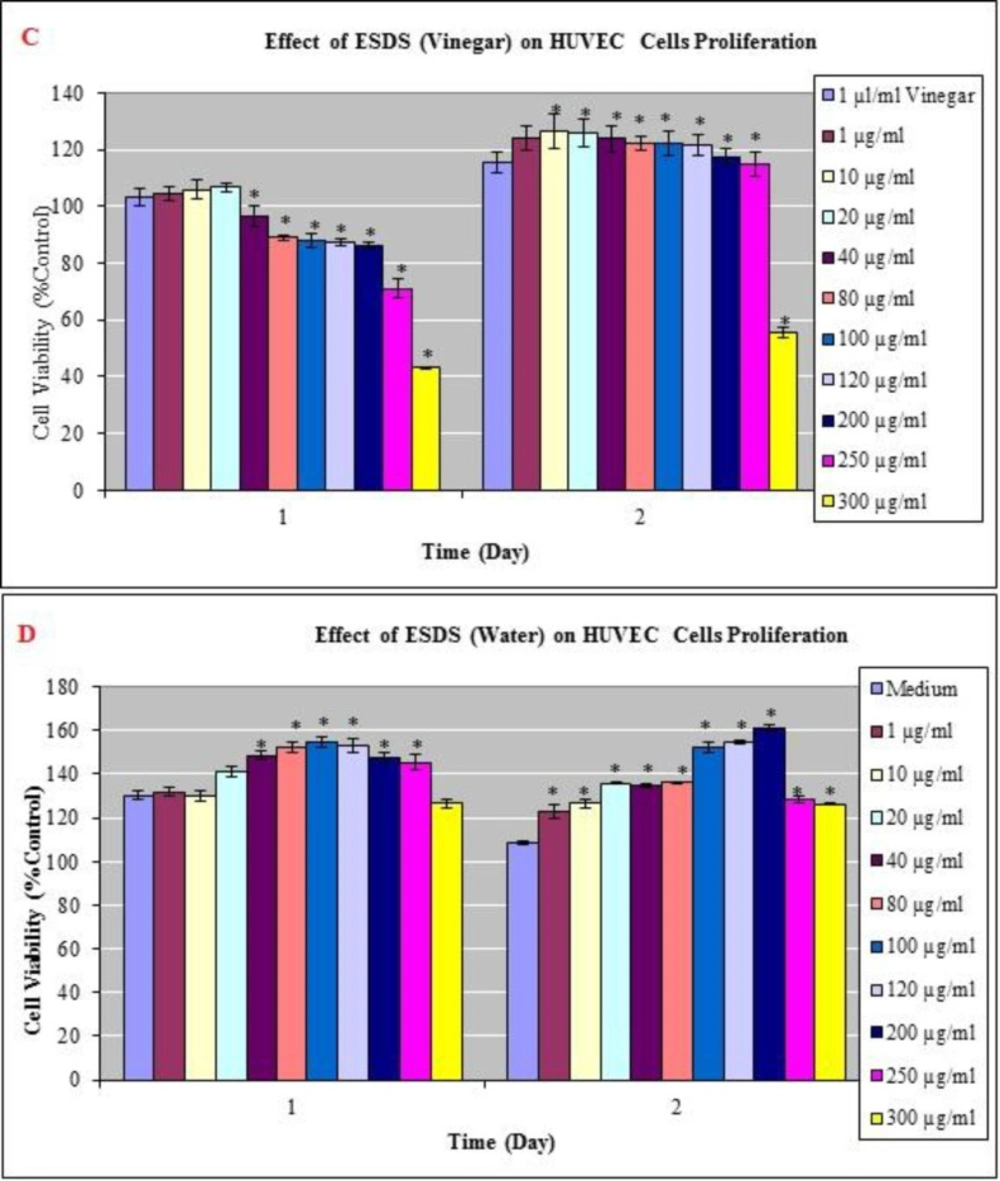
To elucidate the cellular mechanism of D. staphisagria seeds in promoting hair growth in-vitro, HUVECs - a human endothelial cell line - and HaCaT cells - a human keratinocyte cell line - were treated with the vinegar and water extracts. Cells were tested at 24 and 48 h after being treated with vinegar and water extracts of D. staphisagria seeds. Upon both treatments, cell shape had not changed (data not shown) and no overt cytotoxicity was observed in both cell types (Figure 1 and Figure 2). Vinegar and water extracts of D. staphisagria seeds significantly promoted the proliferation of human keratinocyte cells by 137, 139, 143, 149 and 147 % at the concentration of 100, 120, 200, 250 and 300 μg/mL compared with vehicle –treated control, respectively at 24 h (Figure 1A). HUVECs viability remained the same with the control group at the concentration 1, 10, 20 and 40 μg/mL after 24 h. However, the proliferation of endothelial cell was a little lower (10 % of control) at 80, 100, 120 and 200 μg/mL treatment of vinegar extract of seeds. On the contrary, on the second day, proliferation rate was increased about 20% in comparison to the control group in HUVECs (Figure 2). This result suggests that the seeds of D. staphisagria may promote the proliferation of hair follicles and hair growth.
Effects of vinegar and water extracts on HACAT cells (A-B) as determined in the MTT assay. Dose-response curves of the anti-proliferative effect of vinegar and water extract which is prepared from seed of Delphinium staphisagria (ESDS) for MTT assays performed after 24 and 48 h exposures. The results are expressed as the mean_SD. * Indicates significant difference from the control group by the Tukey test (p<0.05
Effects of vinegar and water extracts on HUVECs (C-D) as determined in the MTT assay. Dose-response curves of the anti-proliferative effect of vinegar and water extract which is prepared from seed of Delphinium staphisagria (ESDS) for MTT assays performed after 24 and 48 h exposures. The results are expressed as the mean_SD. * Indicates significant difference from the control group by the Tukey test (p<0.05
Determination of angiogenic potential of the Delphinium staphisagria seeds
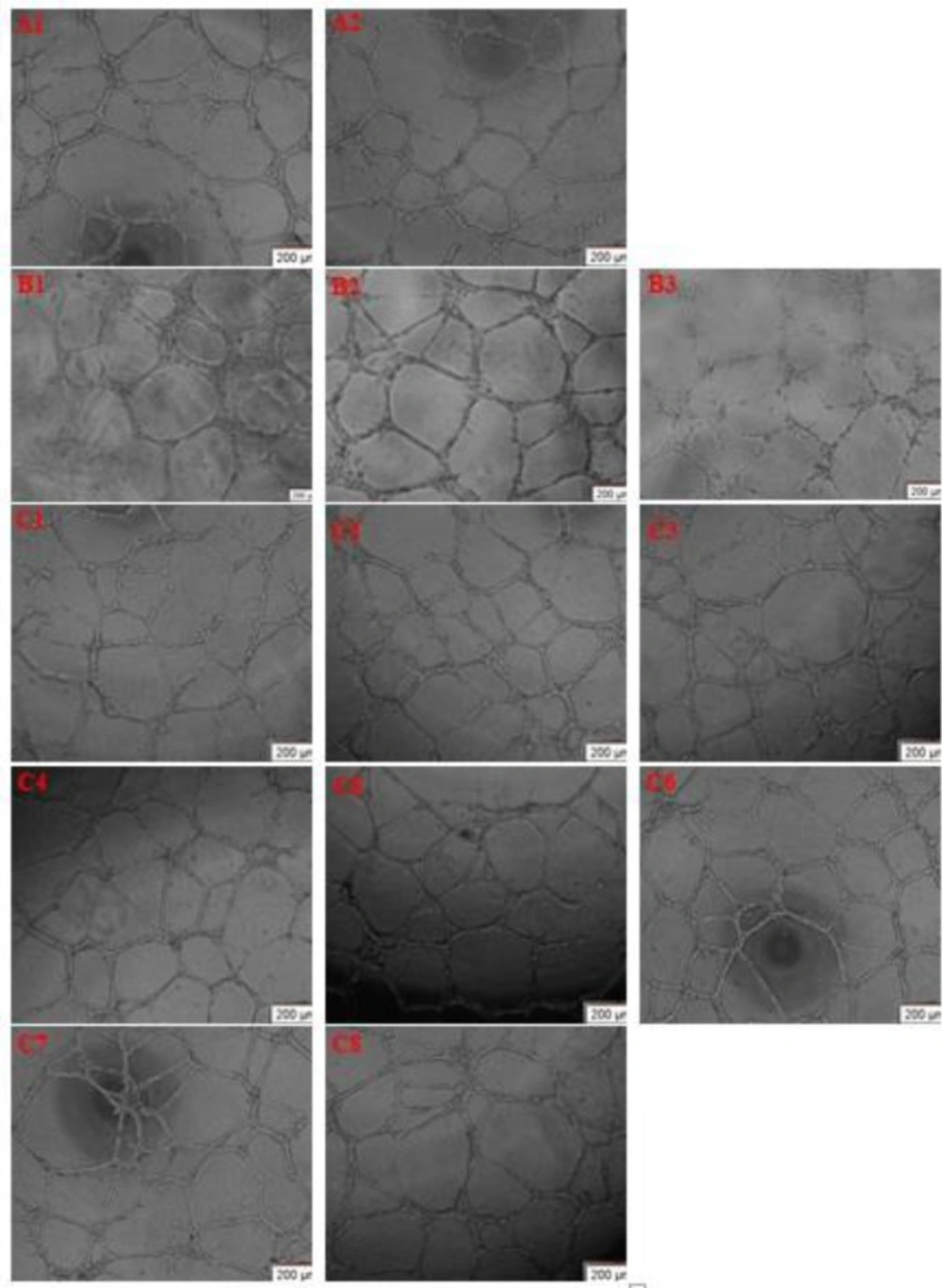
To identify the compounds that induce angiogenesis, we applied matrigel capillary assay in-vitro using HUVECs. Thalidomide at 100, 200 and 400 μM Concentrations was used as the positive control. Thalidomide was found to be a much stronger inhibitor of angiogenesis compared to the control and to the seeds of D. staphisagria. As seen in Figure 3, neither of the extracts exhibited anti-angiogenic activity on HUVECs. ESDS, moderately stimulated both proliferation and migration of HUVECs (i.e., increased tube length and number of branches). We didn’t release any differences between effect of water and vinegar extracts on in-vitro angiogenesis (Water extract’s results not provided).
This results support the traditional information about D. staphisagria seeds’ which has potential hair growth promoting activity.
The effect of ESDS (Vinegar) on HUVECs tube formation. (A1) Control cells and (A2) Vinegar solvent control cells. (B1) HUVECs were treated with Thalidomide 100 μM(B2) Thalidomide 200 μM (B3) Thalidomide 400 μM. (C1) HUVECs were treated with ESDS (Vinegar) 20 μg/mL, (C2) 40 μg/mL (C3) 80 μg/mL (C4) 100 μg/mL (C5) 120 μg/mL (C6) 200 μg/mL (C7) 250 μg/mL (C8) 300 μg/mL. Images shown are representative of independent triplicate assays. Scale bar, 200 μm
Discussion
Hair loss treatment is a global, multibillion dollar industry. To date, two “hair loss drugs” — finasteride and minoxidil — have been approved by Food and Drug Administration (FDA). Nevertheless, their use is limited because of potential side effects such as impotence, abnormal ejaculation, decreased ejaculatory volume, abnormal sexual function, gynecomastia, testicular pain, impairment of muscle growth, severe myopathy, hirsutism, local irridation, itching, dryness, erythema, dizziness and tachycardia, as well as unsatisfactory cure rates (3, 4 and 27-30). On the other hand, natural products with possible health benefits have been more and more appealing and about 1000 kinds of plant extracts have been examined with respect to hair growth (3). For this purpose, we – to our knowledge, for the first time – investigated the angiogenic/antiangiogenic potential and cytotoxicity of D. staphisagria seeds on epidermal keratinocytes (HaCaT) and endothelial cells (HUVECs). These seeds have traditionally been used for a variety of medical purposes. Previous phytochemical studies on the genus Delphinium have reported the presence of diterpenoid alkaloids, flavonoids, sterols and aliphatic acids, respectively, among which diterpenoid alkaloids are considered to be the main characteristic constituents (8, 9, 31 and 32). Burmistrowa et al. (2011), report that the flavonoid derivative astragalin heptaacetate (AHA), which was isolated from the aerial parts of D. staphisagria, induces cell death in human HL-60 leukemia cell line (33). Several studies aimed to identify potential plants that could be harnessed to design new therapies for neurological disorders and neurodegenerative diseases. They reported that the alkaloid delphinine (diterpene alkaloid), which is found in the seeds, has a structure similar to that of aconitine and possesses highly toxic effects on the central nervous system. However, there is no study to explain its hair-promoting effects despite its known insecticide, hydrops, purgative and emetic effects (10, 11 and 34). In our study, we prepared vinegar and water extract from D. staphisagria seeds according to the traditional usage and our results showed that there is no toxicity in either keratinocytes or endothelial cells even at high concentrations.
It is important to block apoptosis of hair follicle keratinocytes and to inhibit exogen phase for therapies of hair loss (17, 35). Additionally, the vascular network is tightly correlated with the hair growth (4) due to the activity of VEGF that is present in hair follicles, sebaceous glands, dermal papilla cell, keratinocytes and also other mesenchymal cells. Given that the growing hair follicle is surrounded by blood vessels (4, 18, 20 and 36-38), improving angiogenesis may be a key factor for the treatment of hair loss or promoting hair growth. It is known that minoxidil efficacy manifests via dilated blood vessels that effectively nourishes the surrounding hair bulbs and induces VEGF expression (39, 40). On top of these effects on endothelial cells, minoxidil stimulates keratinocyte cell proliferation at micromolar doses (41, 42). Based on the hypothesis that angiogenesis during the anagen growth might be associated with endothelial cell proliferation, we applied the matrigel tube formation assay on human endothelial cell to demonstrate the angiogenic potential of D. staphisagria seeds. When the endothelial cells are plated on a layer of basement membrane in-vitro, they attach, migrate and form capillary like structures with a lumen in 12 h (43). We assessed the changes in standard morphological criteria of angiogenesis (microvessel density, vessel calibers, and the number of endothelial cell nuclei) and in number of proliferating endothelial cells qualitatively and quantitatively in defined reference areas throughout the hair cycle (19). Our cytotoxicity results present an idea about the number of proliferating endothelial cells. Up to 250 μg/mL concentration, cell number did not change significantly at 24 h when compared to the control group in vinegar extract. And, importantly, seeds of D. staphisagria at a dose of 1, 10, 20, 40, 80, 100, 120, 200 and 250 μg/mL increased the proliferation of HUVECs to 20% as compared to the control treatment at 48 h. Water extract did not cause inhibition of endothelial and keratinocyte cells proliferation at both time points in all applied concentrations.
While angiogenesis has traditionally been defined as the growth of new capillaries from preexisting vessels, it has recently been proposed that a second type of angiogenesis (“remodeling type”) involves enlargement and elongation of preexisting vessels (43). Our results showed that a capillary-like tube network of HUVECs was formed in the control group, and beyond 20 µg/mL ESDS led to markedly induced tube formation in the same culture conditions (i.e., increased tube length and number of branches). We saw expansion of tubes in the ESDS-treated group in a dose-dependent manner. At non-cytotoxic concentrations of 20 and 300 μg/mL, HUVECs induced capillary vessel formation in culture (Figure 2). We therefore argue that D. staphisagria initially stimulates endothelial cell proliferation and angiogenesis, and then specific signal mechanisms VEGF are transduced to dermal components of the skin and hair growth is stimulated.
Conclusions
One way to discover new drugs is to investigate natural products from medically-used plants. Traditional medicine is very often a valid source for researchers looking for bioactive substances that are potentially useful against many diseases. However, in addition to the limited scientific evidence, there is also a lack of mechanistic understanding on many medicinal ethnopharmacological plants. Our study aims at elucidating Delphinium staphisagria seeds’ pharmacological activities, ethnopharmacology, intellectual property transfer on pharmacological therapies, and toxicity. Our results provide scientific evidence for ethnobotanical claims and point to the gaps required to be filled in for future research.
The chemical constituents and the molecular mechanisms underlying the bioactivity of the Delphinium staphisagria seeds are largely unknown. The potential of this plant can be harnessed to understand the molecular mechanisms governing its therapeutic activity, the active constituents underlying the angiogenic activity, and possibly to uncover new alternatives to the existing therapies for angiogenic diseases and hair repair. Such future studies will be necessary to expand the existing limited therapeutic arsenal for the majority of angiogenesis- and hair-related diseases, especially for those therapies with side effects which limit their effectiveness.