Introduction
Lichens or lichenized fungi are symbiotic organisms consisting of fungi, algae and/or cyanobacteria (1). These associations synthesize a great variety of secondary metabolites that are typical of these groups. As a result of the development of analytical techniques and experimental methods, more than one thousand lichen substances have been identified (2). Lichens and their natural products have been used as medicine, food, fodder, perfume, spice, dyes for different purposes throughout the world (3). Lichen metabolites have several potential biological activities, such as antibiotic, antimycobacterial, antiviral, antitumor, antiherbivore, antiprotozoal, antimutagenic, antiinflammatory, antioxidant, allelochemical, allergenic, analgesic, antipyretic, antiproliferative and cytotoxic effects (1-4).
Lichens produce a large number of phenolic compounds, such as depsides, depsidones, and dibenzofurans. Phenolic compounds have strong antioxidant properties because they act as hydrogen donors and singlet oxygen quenchers and, therefore, have redox capacity (5).
Many lichen species from different parts of the world have been investigated in terms of their potential antioxidant properties, and some have shown very strong antioxidant activities (6-13). However, these studies were made with lichen extracts. In such studies, it is not clear which compounds are responsible for the antioxidant activity. Only, in recent years, a few studies have determined which lichen secondary metabolites have antioxidant effect (5, 14-18).
Burkholder et al. have started pioneering research on lichens as antibacterial agents in 1944 (19, 20). The antimicrobial activity of lichen species was also demonstrated in other studies (21-25). The antimicrobial activity of lichens is variable, depending on the species of lichen, the concentrations of the extract and the tested organisms (26). Most studies have indicated that the lichen species are more effective against Gram-positive bacteria than against Gram-negative ones (27, 28).
The genus Usnea belongs to the family Parmeliaceae (29). The dibenzofuran usnic acid is found in all Usnea species and it is one of the most common, abundant and studied lichen metabolites. Usnic acid as a pure substance has been used in creams, toothpastes, mouthwashes, deodorants and sunscreen products as an active ingredient or as a preservative because of its antimicrobial properties. Usnic acid has many biological activities, such as antibiotic, antibacterial, antifungal, antiviral, antiprotozoal, antiproliferative, anti-inflammatory, analgesic, antipyretic, anti-tumor, antimitotic, antigrowth, antiherbivore and anti-insect effects (30-32). Moreover, the phenolic depsidon stictic acid is found in some Usnea species and has apoptotic, cytotoxic, and antioxidant activities (14, 15, 33 and 34).
The main objective of this study was to determine the stictic and usnic acid content of U. filipendula, U. fulvoreagens and U. intermedia by high performance liquid chromatography-diode array detection (HPLC-DAD) and to examine the efficiency of different solvent systems for the extraction of phenolic compounds. The total antioxidant activity and total phenolic contents of the extracts of three Usnea species were determined by the ABTS and Folin-Ciocalteu methods, respectively. In this study, we also aimed to determine the antimicrobial activity of these species using broth microdilution method.
Experimental
Materials
Trolox [(±)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid], an HPLC grade methanol, ethanol and usnic acid were purchased from Sigma–Aldrich (Steinheim, Germany). Stictic acid was purchased from ChromaDex (California,USA). Analytical-grade acetone, dimethyl sulfoxide (DMSO), tetrahydrofuran and orthophosphoric acid (89%) were purchased from Merck (Merck, Darmstadt, Germany).
Lichen collection and identification
Lichen samples were collected in November 2012 from the trunks of Abies sp. from Uludag Mountain, Bursa, Turkey. The samples were identified by Seyhan Oran using standard methods and information from the literature (34, 35). A voucher specimen of each species (U. filipendula 16444, U. fulvoreagens 16445, U. intermedia 16446) was deposited at the Herbarium of Uludag University (BULU), Bursa, Turkey, for future reference.
Extraction
Air-dried and cleaned lichen materials were ground in a household blender. The lichen samples (1 g) were separately blended with 40 mL of organic solvent (acetone, methanol, or ethanol) at room temperature in the dark for 4 h with a magnetic stirrer. The samples (total volume 40 mL) were separated from the solid matrix by filtration through sheets of filter paper (Whatman No. 1). The extracts were used to determine the total antioxidant activity and total phenolic contents by the ABTS and Folin-Ciocalteu methods, respectively. The organic solvent extracts (5 mL) were evaporated to dryness. Finally, residues were dissolved in 2 mL of DMSO. The DMSO extracts were used for the HPLC analysis.
HPLC
An Agilent 1200 HPLC system (Waldbronn, Germany), consisting of a vacuum degasser, binary pump, autosampler and a diode-array detector was used. Chromatographic separations were carried out using an XBridge C18 (3.5 µm, 4.6 x 250 mm) column from Waters. The mobile phase consisted of 0.25 % orthophosphoric acid and 1.50 % tetrahydrofuran in water (solvent A) and methanol (solvent B). The gradient conditions are as follows; 0-15 min 30-70 % A, 15-30 min 70-100 % A, 30-35 min 100 % A, 35-36 min 100-30 % A, and 36-50 min 30 % A with a total run time of 50 min. The column was equilibrated for 10 min prior to each analysis. The flow rate was 0.25 mL/min, and the injection volume was 10 µL. The data acquisition and preprocessing were performed with ChemStation for LC (Agilent). The monitoring wavelength was 240 nm.
Antioxidant activity assay
The total antioxidant activity of Usnea species was determined with the ABTS method as described by Sarıburun and others (36). The absorbance was measured by spectrophotometry (Varian Cary 50, Australia) at 734 nm against a blank after 6 min. The results were expressed as mg of trolox equivalent (TE) per 100 g of dried weight.
Folin-Ciocalteu method
The total phenolic content as determined by the Folin-Ciocalteu reagent was carried out according to the procedure reported by Sarıburun and others (36). The absorbance was measured by spectrophotometry (Varian Cary 50, Australia) at 750 nm. The total phenols were expressed as mg of gallic acid equivalent (GAE) per 100 g of dried weight.
Bacterial strains
The bacterial strains that were used in this study were Escherichia coli ATCC 25922, Escherichia coli O157:H7, Staphylococcus aureus ATCC 25923, Staphylococcus aureus ATCC 33591 and eight FQ-resistant E. coli isolates.
Determination of Minimum Inhibitory Concentration (MIC)
Broth microdilution testing was performed to determine the MICs of the lichen species according to the guidelines of the Clinical Laboratory Standards Institute (CLSI, 2003). The bacterial cultures were prepared in Mueller-Hinton Broth (MHB) at 37 ºC for 16-20 h. Powdered lichens were extracted with methanol due to common use of methanol as a solvent for in-vitro susceptibility tests. The methanol extracts were dissolved in 20:80 methanol:PBS (phosphate buffered saline) (v/v). Freshly prepared stock solutions were sterilized using 0.20-μm single-use filter units (Minisart, Sartorius Stedim Biotech.). Dilutions ranging from 0.008 to 256 mg/L were prepared in MHB, and inocula with a density equivalent to 0.5 McFarland turbidity were added to tubes containing the extracts’ dilutions. After incubating at 37 ºC for 16-20 h, the MICs were defined as the minimum concentration of extract that inhibited the growth of the organism. The optical densities (ODs) of the cultures were measured at a wavelength of 595 nm (Bio-Rad, iMark).
Results
Identification of Stictic and Usnic acids in Usnea species
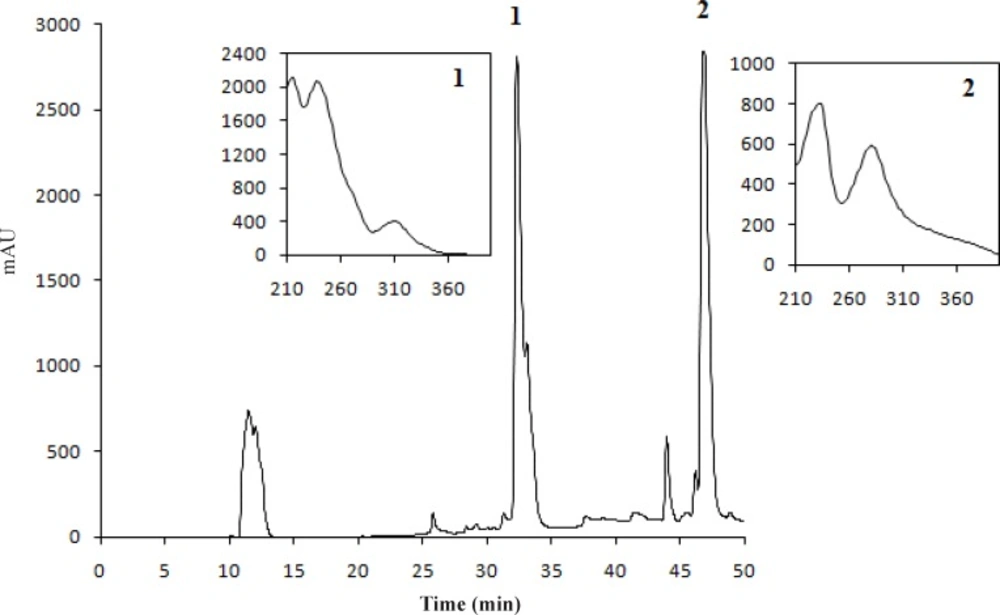
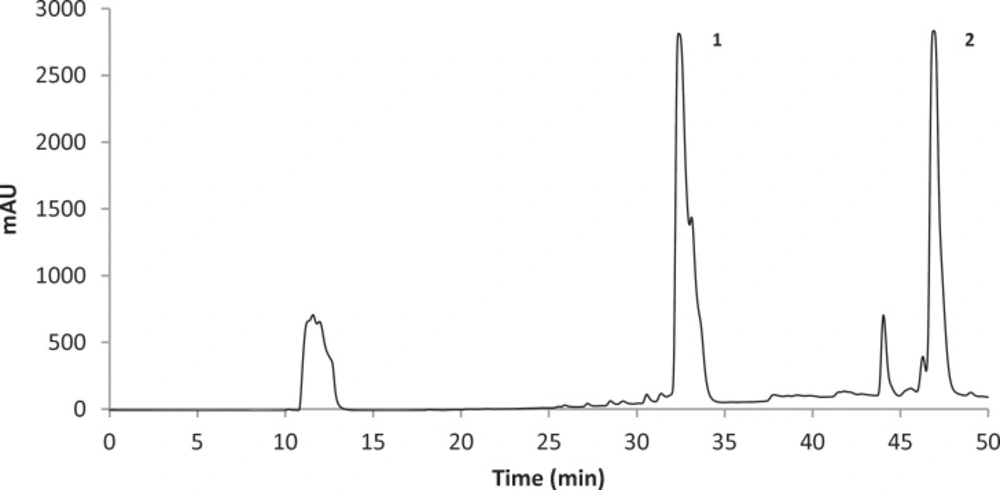
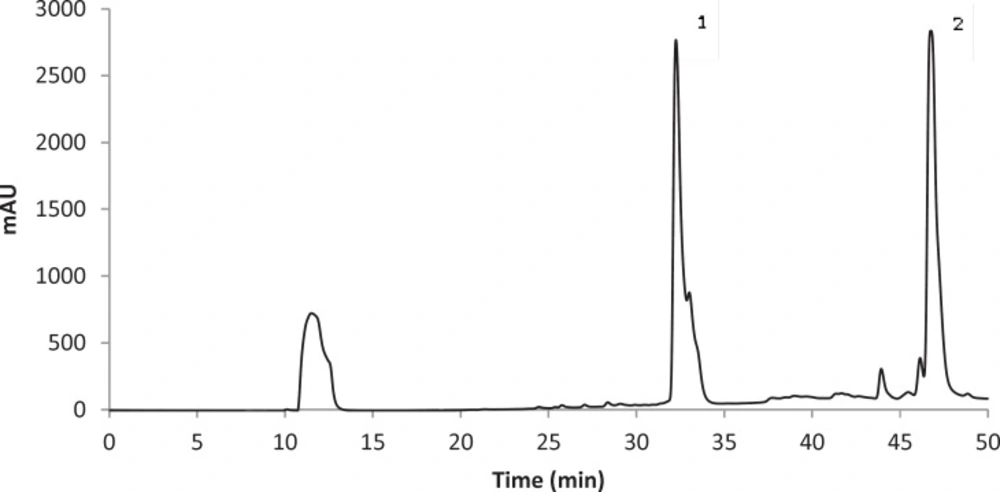
The concentrations of stictic and usnic acids in the three different extracts of U. filipendula, U. fulvoreagens, and U. intermedia were determined by HPLC-DAD, and the results are shown in Table 1. Identification of these compounds was achieved by comparison of their retention time values with the standard substance purchased from ChromaDex and Sigma-Aldrich. The HPLC chromatograms of the acetone extracts of U. filipendula, U. fulvoreagens and U. intermedia are shown in Figures 1-3.
| Extraction solvent | Stictic acid | Usnic acid | |
|---|---|---|---|
| Usnea filipendula | Acetone | 9.85±0.49 | 1.10±0.16 |
| Ethanol | 5.72±0.06 | 0.97±0.03 | |
| Methanol | 5.37±0.48 | 1.07±0.09 | |
| Usnea fulvoreagens | Acetone | 7.60±0.28 | 1.19±0.01 |
| Ethanol | 5.33±0.09 | 1.01±0.01 | |
| Methanol | 4.77±0.51 | 1.04±0.07 | |
| Usnea intermedia | Acetone | 5.72±0.14 | 0.97±0.05 |
| Ethanol | 4.04±0.18 | 0.72±0.02 | |
| Methanol | 2.98±0.28 | 0.94±0.04 |
The highest concentrations of stictic and usnic acids were found in the acetone extract of U. filipendula (9.85±0.49 mg of stictic acid/g of dried lichen) and U. fulvoreagens (1.19±0.01 mg of usnic acid/g of dried lichen) respectively. The smallest concentrations of stictic and usnic acids were found in the methanol extract (2.98±0.28 mg of stictic acid/g of dried lichen) and ethanol extract (0.72±0.02 mg of usnic acid/g of dried lichen) of U. intermedia. The stictic acid concentrations that were obtained by the HPLC method indicate that the order of solvent efficiency is acetone > ethanol > methanol, while the usnic acid concentrations that were obtained by the HPLC method indicate that the order of solvent efficiency is acetone > methanol > ethanol for the three Usnea species. The solvents with a relatively lower polarity were more efficient in general in extracting phenolic compounds in the three Usnea species. Because of the different solubility of the lichen substances in different solvent, hexane, diethyl eter, acetone and methanol were used for the extraction of lichen substances. Acetone is very useful solvent for the extraction, as the most lichen substances are soluble in this solvent. Also acetone was used for the extraction of lichen acids from Usnea species in the literature (37).
Antioxidant activity and total phenolic content of Usnea species
The antioxidant activity and total phenolic contents of the three Usnea species are displayed in Tables 2 and 3. The highest antioxidant activity and total phenolic contents were found in the methanol extract of U. fulvoreagens (181.0±4.4 mg of TE/100 g of dried lichen) and the acetone extract of U. filipendula (329.7±18.5 mg of GAE/100 g of dried lichen), respectively. The antioxidant activity measured by the ABTS method indicate that the order of solvent efficiency is methanol > acetone > ethanol except for U. intermedia. The total phenolic contents as determined by the Folin method indicate that the order of solvent efficiency is methanol > ethanol > acetone except for U. filipendula. As seen from these results, in general, methanol could recover the highest yield of antioxidant activity and total phenolic contents.
| Extraction solvent | U. filipendula | U. fulvoreagens | U. intermedia |
|---|---|---|---|
| Acetone | 125.2±6.4 | 73.4±0.5 | 52.2±2.0 |
| Ethanol | 65.4±0.5 | 61.0±1.7 | 115.8±11.3 |
| Methanol | 174.8±6.9 | 181.0±4.4 | 141.6±6.0 |
| Extraction solvent | U. filipendula | U. fulvoreagens | U. intermedia |
|---|---|---|---|
| Acetone | 329.7±18.5 | 135.5±10.4 | 170.2±1.1 |
| Ethanol | 197.4 ±16.0 | 174.4±1.3 | 194.7±2.2 |
| Methanol | 291.5±12.1 | 229.9±14.0 | 239.7±0.3 |
Validation of the the analytical method
The linearity of the HPLC-DAD method was investigated for phenolic compounds in the range of 1-100 mg/L for stictic acid and 1.4-140 mg/L for usnic acid at nine concentration levels. Two calibration plots with R2 ≥ 0.999 correlation coefficient were obtained by reporting the peak areas as a function of the concentrations of each phenolic compound (Table 4). The validation of the quantitative determination of the phenolic compound concentration in the Usnea samples was performed by estimating the limits of detection (LOD, 3 s/m), limits of quantification (LOQ, 10 s/m) and recovery (%) of stictic and usnic acids (Table 4), where s is the sample standard deviation for the replicates, and m is the slope of the calibration curve. The LOD ranged from 0.49 to 0.039 mg/L, and the LOQ ranged from 1.64 to 0.13 mg/L for stictic and usnic acids, respectively. The extraction efficiency of the lichen standards of stictic and usnic acids was evaluated by spiking the samples with the mixture of standards and extracting using acetone, ethanol and methanol. The recovery study was carried out only for usnic acid as identified by HPLC-DAD. The mean percentage recoveries ranged from 92.22±1.72 to 96.37±2.13 (Table 4). All of the other recoveries are within the experimental error range. In the calculation of the final results, the recoveries of the pure phenolic standards were considered.
| Validation parameters | Stictic acid | Usnic acid |
|---|---|---|
| LOD (mg L-1) | 0.490 | 0.039 |
| LOQ (mg L-1) | 1.640 | 0.130 |
| R2 | 0.999 | 0.999 |
| Recovery (%) | ||
| Acetone | 96.37±2.13 | 92.22±1.72 |
| Ethanol | 95.97±1.77 | 94.34±1.56 |
| Methanol | 95.07±1.61 | 95.38±2.38 |
Antimicrobial activity of the lichen extracts
In this study, the broth microdilution method was used to test for antimicrobial susceptibility. The MICs of the methanol extracts of the lichen species ranged from 64 µg/mL to ≥ 512 µg/mL for all of the bacterial strains that were tested in this study (Table 5). The highest antimicrobial activity was observed for the methanol extracts of U. filipendula, U. fulvoreagens and U. intermedia against E. coli E245 and for the methanol extracts of U. filipendula and U. intermedia against E. coli O157:H7 with 64 µg/mL MIC.
| Isolates ID | MIC (µg/mL) | ||
|---|---|---|---|
| U. filipendula | U. fulvoreagens | U. intermedia | |
| E. coli E101 | 512 | ≥512 | 256 |
| E. coli E103 | 128 | 128 | 128 |
| E. coli E121 | 128 | 128 | 128 |
| E. coli E224 | 128 | 128 | 128 |
| E. coli E245 | 64 | 64 | 64 |
| E. coli E246 | 128 | 128 | 128 |
| E. coli E248 | 128 | 128 | 128 |
| E. coli E300 | 128 | 128 | 128 |
| E. coli 25922 | 128 | 512 | 128 |
| E. coli O157:H7 | 64 | 512 | 64 |
| S. aureus 25923 | 128 | 512 | 128 |
| S. aureus 33591 | 256 | 256 | ≥512 |
Discussion
The depsidone stictic acid is an aromatic organic compound and a central subject of medical research. Stictic acid has a significant apoptotic effect (33). To the best of our knowledge, this study is the first to establish the stictic acid concentration in three different solvent extracts of U. intermedia and U. filipendula.
Usnic acid is found in several lichens and is especially abundant in the genera Alectoria, Cladonia, Usnea, Lecanora, Ramalina and Evernia. The biological activity of usnic acid has been studied previously (32, 38). Many lichens and extracts containing usnic acid have been utilized for medicinal, perfumery, and cosmetic areas (30). Our results indicate that the acetone extracts of three species of Usnea could be used for these applications because of their contents of usnic acid. The contents of usnic acid in our species were lower than the content of usnic acid with these of Usnea longissima, Usnea hirta and Usnea rigida in the literature (37).
Folin Ciocalteu analysis and ABTS data evidenced no correlation between the total phenolic contents and the antioxidant activities of the Usnea extracts. The presence of phenolic groups in lichen metabolites is considered as a key element for their antioxidative effect, but antagonistic or synergistic effects of different chemicals when interacting with each other should be considered. Moreover, only a few studies have considered the possible interactions between phenolics, whereas a potent regeneration of an antioxidant by another antioxidant can increase or decrease the activity of a mixture of antioxidants (39).
Previous reports on the antimicrobial properties of lichens have demonstrate a low antimicrobial activity against Gram-negative bacteria, but in this study, FQ-resistant E. coli isolates (except for E101) were sensitive to the three Usnea species as reported previously (22, 37, 40). Furthermore, our results demonstrate that there is no difference between the antimicrobial sensitivity of S. aureus strains and E. coli strains and isolates.
Conclusion
In conclusion, our findings indicate that the three Usnea species could be used as antioxidant and antimicrobial agents. Lichen metabolites are poorly studied as a new source of active compounds. Therefore, further studies are required to isolate the lichen substances that exhibit potent biological activity.