Introduction
Teratogenic exposures can cause an embryo or fetus defects. Teratogenicity depends upon the ability of the agent to cross the placenta. The embryo is most susceptible to teratogenic agents during periods of rapid differentiation. (1, 2). The morphological type of abnormality depends on the developmental stage at which the drug reaches the fetus and may be drug-specific. Whereas obvious anatomical defects result from disturbed organogenesis during early pregnancy, more subtle functional changes may occur due to intrauterine exposure after the first trimester. This appears to be most likely in the nervous system because of this system's continued histogenesis after the first trimester (3).
Phenobarbital is a phenobarbiturate used as a sedative, anticonvultant or hypnotic with the doses prescribed. The combined use of it with other antiepileptics especially diphenylhydantoin increase its teratogenic effects. Phenobarbiturate can cause malformations such as facial dysmorphism, fetal hydantoin syndrome, with small saddle nose, large mouth with no philtrum, synophrys, and deformed helix. The possible teratogenic effect of phenobarbital in the offspring of pregnant women in the dataset of the Hungarian Case-Control Surveillance System of Congenital Abnormalities was evaluated, and an association of oral phenobarbital treatment with a higher risk of structural birth defects, that is, congenital abnormalities (CAs) was not found. Several studies show that the stimulation of maternal immune system can decrease or prevent drug- induced embryonic abnormalities (4-10).
There are controversial results regarding the teratogenic effect of phenobarbital, but the FDA classified the fetal risk of phenobarbital in the pregnancy category D (There is positive evidence of human fetal risk, but the benefits of its use in pregnant women may be acceptable despite the risk) (11).
The spectroscopic study of biological cells and tissue is an active area of research with a primary goal being to explain how accurately FTIR can determine whether cells or tissue are cancerous or not. FTIR is a potential tool for noninvasive optical tissue diagnosis. In recent years, its application in biological studies and particularly clinical investigations related to malignancy and cancer detection using spectroscopy techniques has been increased by both the clinical and non-clinical researchers (12-14). Since tissue is microscopically heterogeneous, microspectroscopy is the sampling mode most capable of representing accurately tissue complexity in histopathologic determinations. The development of clinical protocols for the routine examination of tissue histology or localized tumors using IR microspectroscopic methods has not been possible due to several related issues (15-18). This study was aimed to measure the possibility of FTIR- MSP application for the recognition and discrimination of teratogtenecity with respect to control during the fetus growth and its relationship with morphological variations.
Experimental
Tissue preparation
Adult mice (10-12 weeks) weighting 20 g were obtained from Razi institute, Iran. The mice were fed with a standard diet with water ad libitum and kept in a room with controlled light (12:12, dark: light), temperature (22±10 ₒC), relative humidity (40-50%) and ventilation (15 air changes per hour). They were allowed to adapt to their environment for 1 week prior to the experiments. The mice were randomly mated and for emphasis of pregnancy their vaginal plaque were assessed after mating. Then, they were separated as control (3 mice) and phenobarbital treated groups (3 mice) (120 mg/Kg/day IP based on reference 19) on gestation day 9 of pregnancy. Pregnant mice were sacrificed and dissected on day 15th of gestation and morphological and histological studies on the fetus were carried out (20). Measurement of fetus weight accomplished by digital balance and Crown-Rump (C-R) lengths accomplished by coils was done.
Also, after tissue fixation with bouin fixative solution, fetus sections at a pre-defined thickness of 10 µm were performed. Slices were either thaw-mounted on a 1 mm thick KBr window for IR microscopy and were mounted on conventional glass slides for staining with haematoxylin (H) and eosin (E) for studying of abnormality in fetus by light microscopy.
FTIR micro spectroscopy
FTIR measurements were performed in the absorbance mode.WQF-510 Fourier transform spectrometer (Rayleigh Optics, China) was equipped with a KBr beam splitter and a DLaTGS (Deuterated Lanthanide Triglycine Sulphate) detector and use of KBr window for µMAX IR microscope (PIKE Technologies, USA). The spectra were scanned in the mid-IR range from 4000 to 400 cm-1 with a resolution of 4 cm-1. 100 scans were coded for each spectrum and the spectra were ratio against the background spectrum.
Data processing and analysis
The data were analyzed using routines of the Main FTOS IR software. The spectra were normalized after the baseline correction of the entire spectrum. The spectra were recorded from several sites on the liver and brain tissues of mice fetus sections and an average spectrum from the all spectra (30 spectra for control and 30 spectra for treated tissuess) was computed. Second order derivatives were also calculated. Calculation of the second derivatives enhanced spectral features and also compensates for baseline shifts. From normalized absorbance spectra various spectral parameters were calculated and plotted against the x and y pixel coordinates.
Statistics
The data from the fetus weight and the fetus C-R length were analyzed by using Graphpad Prism© software. The statistical results of the phenobarbital treated fetus weight with the control fetus weight were compared with Students t-test (p<0. 01). Also, the statistical results of the phenobarbital treated fetus C-R with the control fetus C-R were compared with using Students t-test (p<0. 01). Results were presented as mean± SD.
Results and Discussion
Morphologic studies
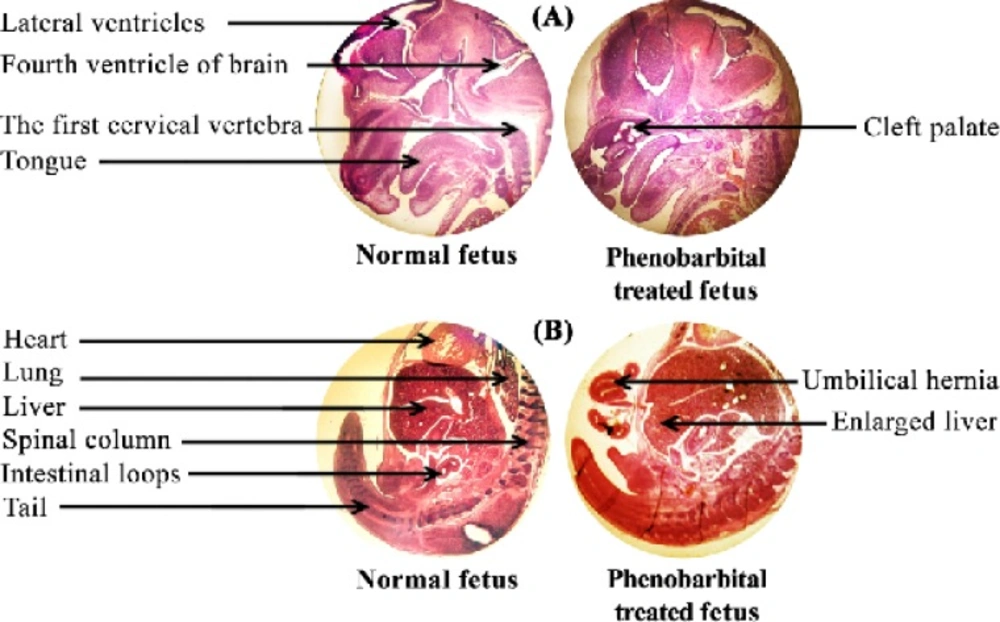
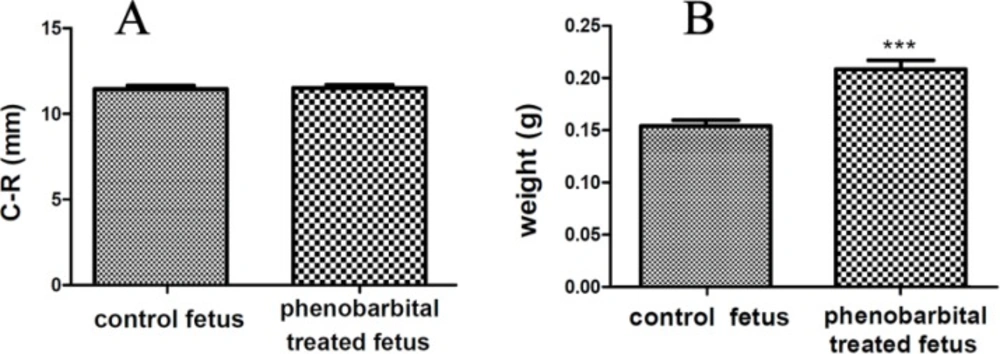
Figure 1 shows the H & E stained sections of a normal and phenobarbital treated mice fetus liver (A) and brain (B). Clearly, the size of phenobarbital treated fetus and especially its liver is larger than the normal fetus. Cleft palate and umbilical hernia were seen in Phenobarbital treated fetus (Figure 1 (A) & (B)). In this study, the C-R length of phenobarbital treated fetus was more than the normal fetus, but there wasn't any significant difference between them (control group C-R mean, 10.54±0.76 and treated group C-R mean, 10.53±0.66) (p<0.01) (Figure 2 (A)). The weight of phenobarbital treated fetus was more than that of normal fetus and there was a significant difference between them (control group weight mean, 0.154±0.018 and treated group weight mean, 0.209±0.029) (p<0. 01) (Figure 2 (B)).
FTIR studies to determine compositional, structural and dynamical changes in phenobarbital treated mice fetus liver
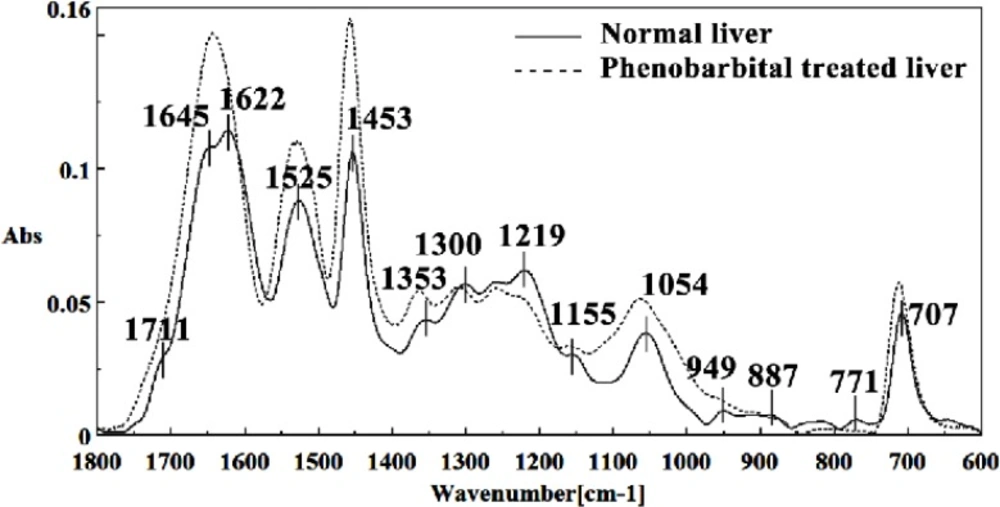
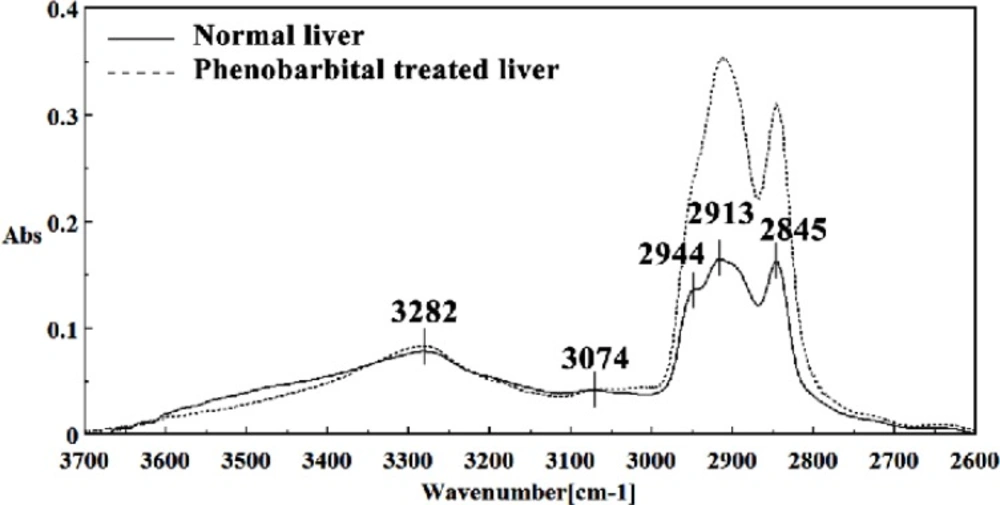
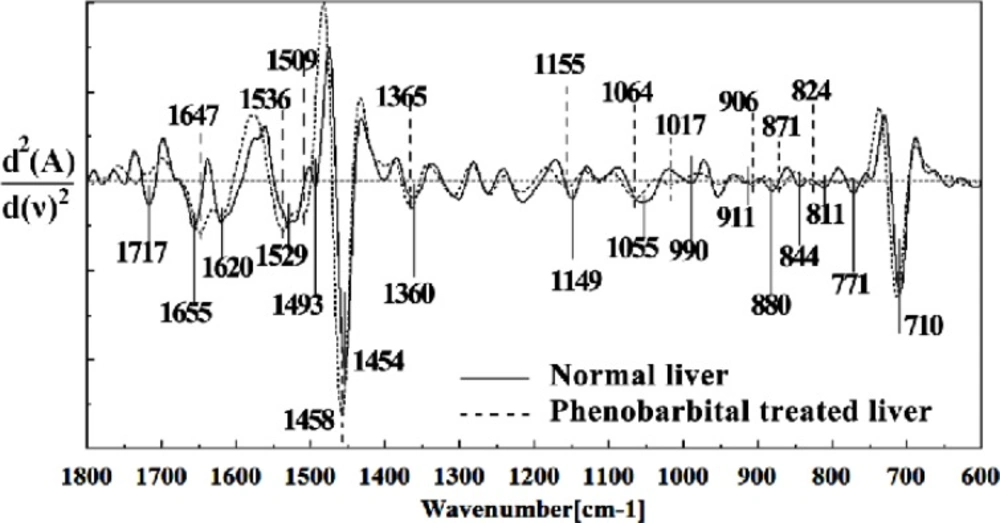
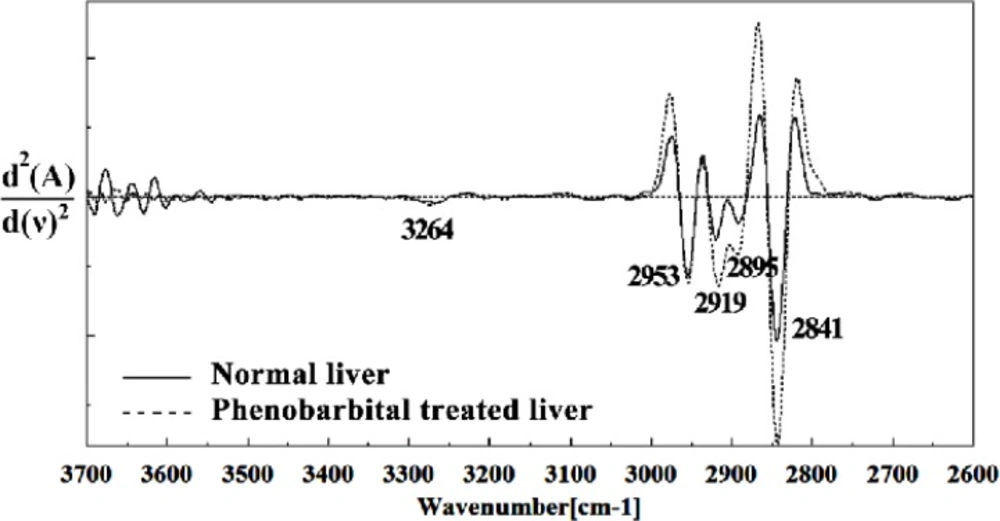
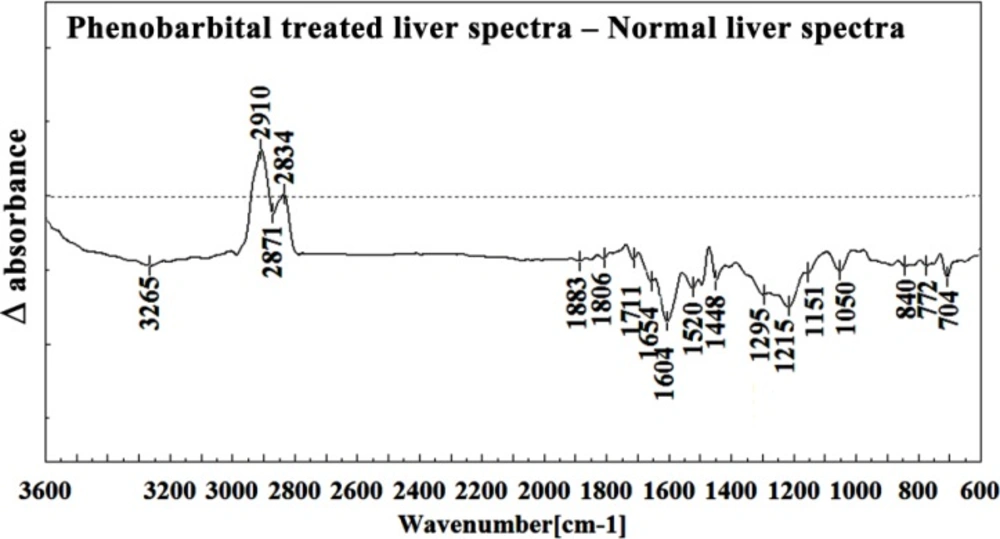
In the present study, the effect of phenobarbital treatment at 120 mg/Kg/day dose on 9th day of pregnancy in mice fetus was investigated at molecular level by using FTIR microspectroscopy. Figures 3, 4, 5, 6 and 7 illustrated the typical IR spectra, 2nd derivative and also difference of the liver tissues spectra of mice fetus exposed to phenobarbital minus the control liver tissues spectra. The information contained in this IR absorption spectrum originates from many different types of biomolecules in the tissue, including proteins, lipids, carbohydrates, and nucleic acids.
The intensity and frequency of the amide I bands at around 1645 cm-1 and 1622 cm-1 in treated tissue were increased, shifted and also converted to one peak instead of two peaks compared to control tissue, mainly owing to the Amide I, C5 methylated cytosine, C=O, stretching C=C uracyl, NH2 (1645 cm-1) and peak of nucleic acids due to the base carbonyl stretching and ring breathing mode (1622 cm-1).
Moreover, the intensity and frequency of the amide II bands at around 1453cm-1and 1525cm-1 in treated tissue were increased and shifted compared to control tissue, mainly owing to asymmetric methyl deformation and Stretching C=N, C=C, respectively. The intensity and frequency of the bands at around 1353 cm-1 was increased and shifted compared to control tissue, mainly owing to Stretching C-O, deformation C-H, deformation N-H, the intensity at 1300 cm-1 was shifted owing to Deformation N-H cytosine, the intensity at 1256 cm-1 and 1219 cm-1 were reduced and shifted compared to control tissue in treated tissue PO-2 asymmetric (phosphate I) (1256 cm-1) and PO2- asymmetric vibrations of nucleic acids when it is highly hydrogen-bonded Asymmetric hydrogen-bonded phosphate stretching mode (1219 cm-1).
The absorption band at around 949 cm-1 in treated tissue resulted from Symmetric stretching vibration of v1PO4 3- (phosphate of HA) was approximately disappeared. The intensity of Left-handed helix DNA (Z form) at 841 cm-1 , a band at 817 cm-1 in the treated tissue, corresponding to Ring CH deformation were reduced. The absorption band at around 771 cm-1 in treated tissue resulted from Guanine in a C3´ endo/syn conformation in the Z conformation of DNA was reduced and shifted in treated fetus. The absorption band at around 707 cm-1 owing to the Out-of-plane bending vibrations was shifted and increased in treated fetus. A band at 1155 cm-1 in treated tissue, corresponding to the C-O stretching vibration was increased and shifted.
The absorption band at around 1711 cm-1 due to C=O (the region of the bases) in treated tissue was disappeared. The intensity of the absorption band at around 1054 cm-1 due to from phospholipid phosphate and partly from oligosaccharide C-OH bonds phosphate ester in treated tissue was gradually increased and shifted.
The intensities of the absorption bands near 2944 cm-1 (stretching C-H), 2913 cm-1 and 2845 cm-1 (Stretching vibrations of CH2 and CH3 of phospholipids, cholesterol and creatine) in treated tissue were increased and shifted compared to the untreated tissue.
FTIR studies to determine compositional, structural and dynamical changes in phenobarbital treated fetus brain
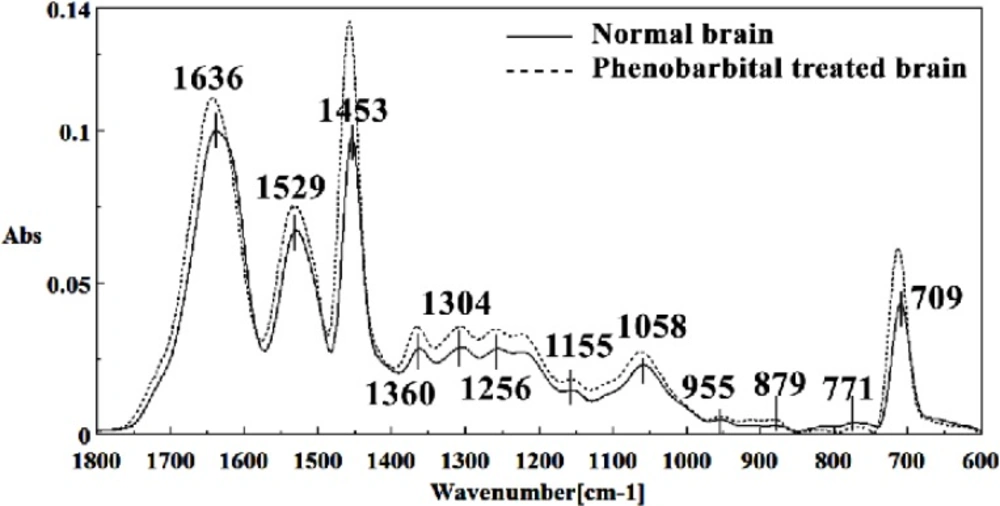
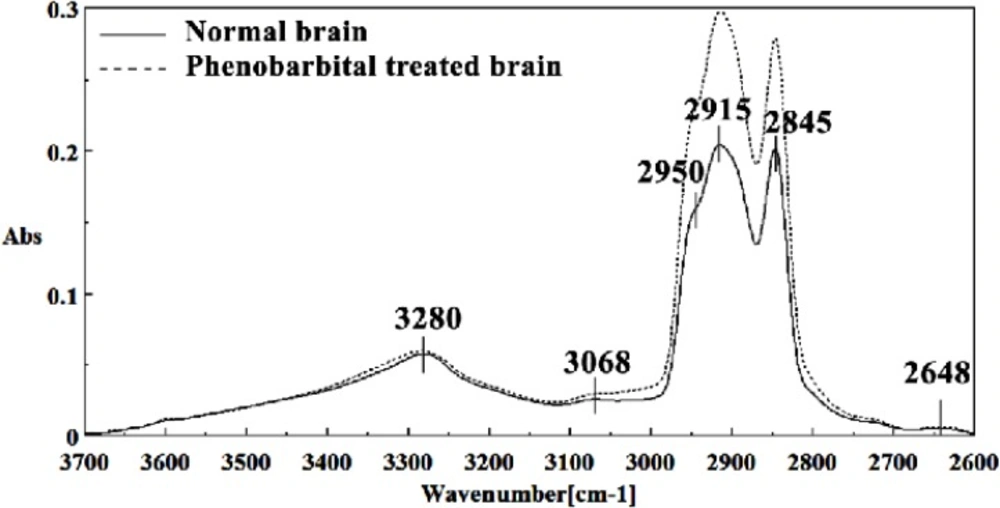
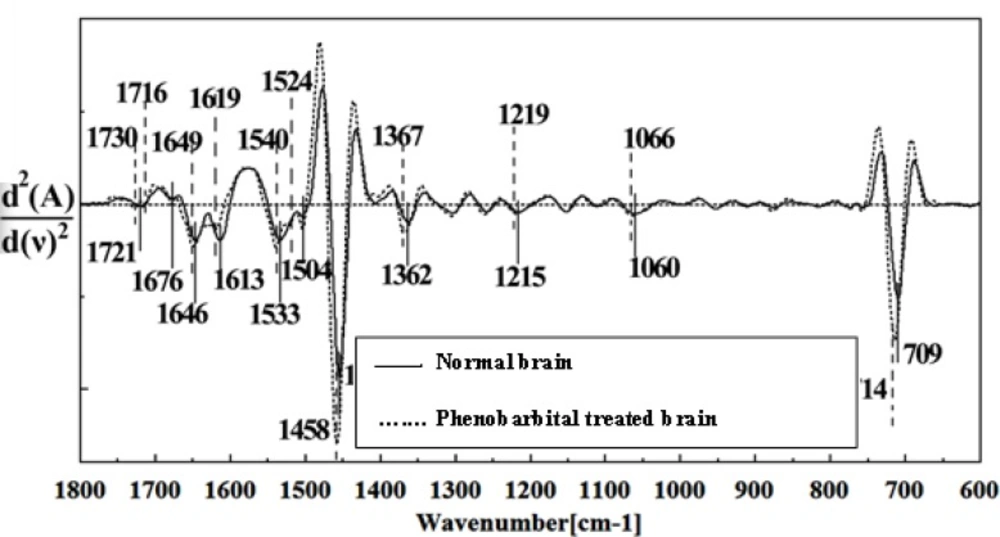
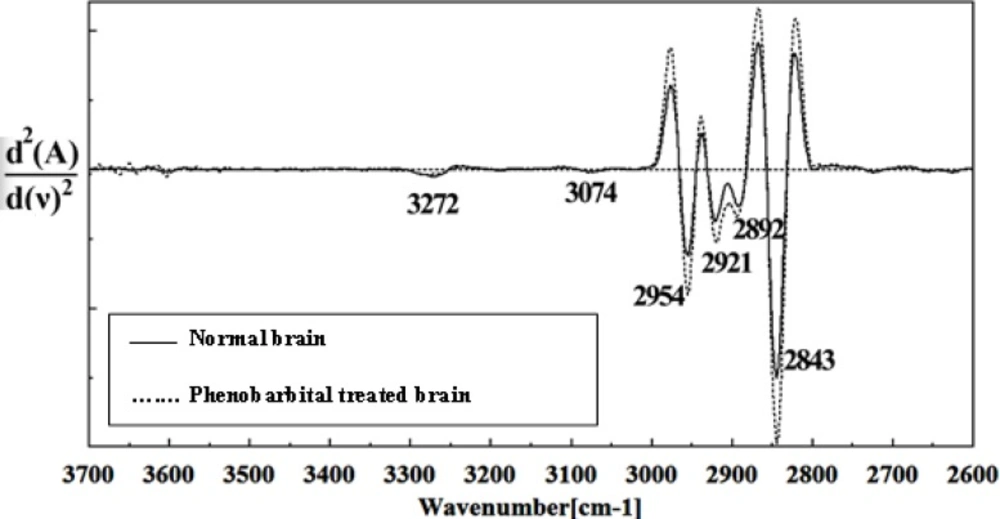
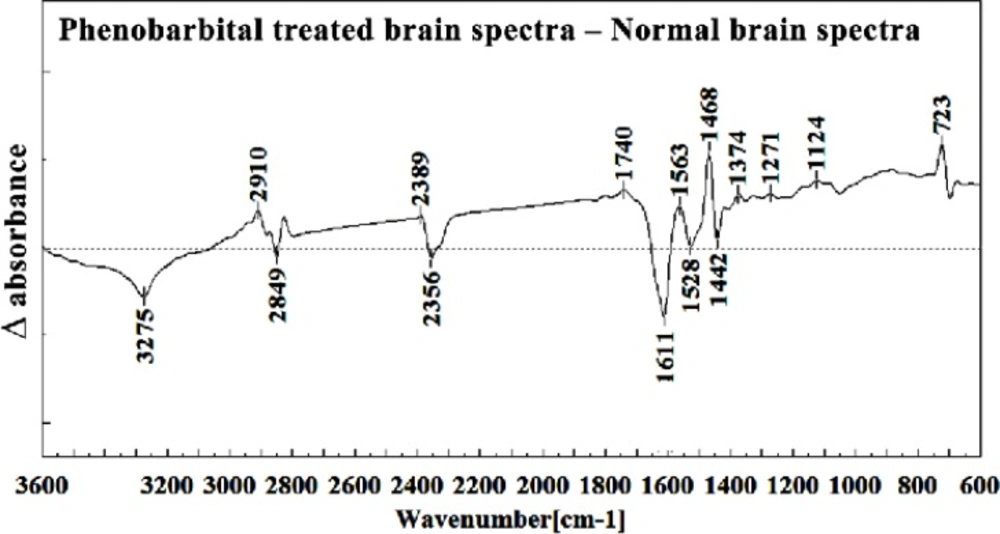
Figures 8, 9, 10, 11 and 12 illustrated the typical IR spectra, 2nd derivative and also the difference of brain tissues of mice fetus exposed to phenobarbital minus the control brain tissues.
The intensity and frequency of the amide I band at around 1636cm-1 in treated tissue were increased and shifted compared to untreated tissue, mainly owing to the β-sheet structure of amide I. Also this band was converted to two bands in treated tissue. Moreover, the intensity and frequency of the amide II bands at around 1453 cm-1 and 1529 cm-1 in treated tissue were increased and shifted compared to untreated tissue, mainly owing to Asymmetric methyl deformation and C=N adenine, cytosine, respectively. The intensity and frequency of the bands at around 1360 cm-1, 1304 cm-1, 1256 cm-1 and 1223 cm-1 in treated tissue were increased and shifted compared to untreated tissue, mainly owing to Stretching C-O, deformation C-H,deformation N-H (1360 cm-1), Amide III(1304 cm-1) PO-2 asymmetric (phosphate I) (1256 cm-1) and PO2- asymmetric (phosphate I) (1223 cm-1). The intensity of the absorption band at around 1058 cm-1 due to 2-Methylmannoside, Oligosaccharide C-OH stretching band and Mannose & mannose-6-phosphate in treated tissue was gradually increased and shifted.
The absorption band in treated tissue at 911 cm-1 was increased and shifted owing to the Phosphodiester stretching bands region (for absorbance due to collagen and glycogen). The absorption band at around 955 cm-1 in treated tissue resulted from Symmetric stretching vibration of v1 PO43- (phosphate of HA), and the intensity was increased and shifted compared to untreated tissue. The intensity of C3´ endo/anti (A-form helix) conformation at 879 cm-1 was increased and shifted and the intensity of the ring CH deformation at 810 cm-1 in treated tissue was reduced and shifted. The absorption band at around 771 cm-1 in treated tissue resulted from Guanine in a C3´ endo/syn conformation in the Z conformation of DNA was reduced in treated fetus. The absorption bands at around 709 cm-1 owing to the Out-of-plane bending vibrations was increased and shifted in treated fetus. A band at 1155 cm-1 in treated tissue, corresponding to the C-O stretching vibration was increased and shifted.
Conclusion
The teratogenicity of Phenobarbital has been extensively presented in many references through the morphological and histological investigations. Studies on rodents exposed to anticonvulsants drugs have shown a widespread apoptotic neurodegeneration in the fetus brain (21). The exact molecular alterations of these defects have not yet been explained. Biospectroscopy is the main technique used for some decades to solve this problem. This technique has proven itself in the differential diagnosis of cancer from normal tissues at the molecular level (22). Here, by using the FTIR-MSP, the result of precise investigation at the chemical bands of mice fetal tissue biomolecules and their alterations after exposure to a D category medication is presented for the first time. As shown in the result section, Phenobarbital induces lots of changes in the mouse fetus at molecular levels in both very important and critical tissues of brain and liver. The chemical characteristics and conformational features of almost all parts of cells are altered dramatically from the lipid bilayer matrix of the cell membrane to the nucleus DNA. Their modifications will represent conformational changes in the proteins which will be presented as metabolism malfunction as well as the structural proteins to be presented in the histology malformation. In any case, the most important changes of the brain tissue is on the β structure of proteins due to the amide I bands being appeared in a lower frequencies specially at 1636 cm-1. This might be as a result of a looser protein structure, particularly to be important in the structural proteins. On the other hand, extensive effects on the DNA structural changes are obvious for the Phenobarbital treated liver tissues. Not only a significant alteration in the N-H band of the cytosine, but also the C-O and phosphate backbone of DNA represented at 1155 cm-1 and 1536 cm-1 are dramatically affected. Although more detail and statistical power are needed to prove and elaborate on these findings, but with even these initial investigations, dramatic structural changes in the brain, and gene expression modifications in the liver of treated tissues are obvious as the result of Phenobarbital exposure to the mouse fetus. These findings might very much introduce a powerful tool for the teratogenic investigation on phenobarbial effects on fetus and its uses in pregnant women. FTIR can be assumed as a potentially useful technique in understanding fetus changes after using different drugs. More studies are ongoing and results will be published in the future.