Introduction
There are growing interests in use of plants as natural antimicrobial agents because they do not induce antibiotic resistance which is common in the synthetic antibiotics. Securigera securidaca (L.) Deg. & Dorf. (Fabaceae) is one of three species of this genus which grows in Iran (1). Rosa damascena Mill. (Rosaceae) is a small plant with aromatic flower which appears in spring (2). Nowadays, R. damascena is the principle species cultivated for Rose water and attar in central part of Iran (Kashan), India and Bulgaria (3). Tripleurospermum disciforme (C.A. Mey) Schultz Bip., a genus of Asteraceae, is one of the native plants of Europe and western Asia (4). It was grown in many parts of Iran. These three plants had many traditional and folk uses in Iran but there were a few reports about antimicrobial effects of them.
The people in the south of Iran used oral administration of the seeds of S. securidaca for hypoglycemic effects. S. securidaca extract significantly reduced glucose level in diabetic animals by a mechanism different from sulfonylurea agents (5, 6).
R. damascena has some benefits such as cooling, soothing, astringent and anti inflammatory effects (7). Its extract and essential oil showed antioxidant and antibacterial properties (8-10). Rose water is a natural healer for various skin problems and a skin care in folk medicine of Iran. It is an important ingredient in many body creams and cosmetics in the world due to its pleasant fragrance and useful properties.
T. disciforme was used as anti inflammatory, anti spasmodic, anti septic, carminative and as a hair color (11, 12).
The objective of present research is to evaluate antimicrobial effects of S. securidaca, R. damascena and T. disciforme extracts and isolation and identification of compounds of T. disciforme.
Experimental
Plant material
The seeds of S. securidaca, petals of R. damascena and top flowered of T. disciforme were collected in September, May and July 2011 around the Fars, Gilan and Tehran Provinces of Iran, respectively. The plants were dried in shade and powdered. A voucher specimen of each plant is deposited at Herbarium of Faculty of Pharmacy, Tehran University of Medical Sciences.
Preparation of extracts
The powder of dried seeds of S. securidaca, petals of R. damascena and top flowered of T. disciforme (400 g of each sample) were macerated separately with 80% methanol at room temperature in a percolator, then solvents concentrated in vacuum to give gummy residue (crude extract). The crude extract of S. securidaca was re-extracted with petroleum ether, chloroform and methanol to achieve different fractions. The concentrated extracts and fractions were kept at 4 ºC prior to antimicrobial tests.
Microorganisms and media
The various organisms were used as standard strains in this study, include Staphylococcus aureus ATCC6538, Staphylococcus epidermidis ATCC12229, Bacillus subtilis ATCC6633 and Bacillus cereus ATCC1274 as Gram positive bacteria; Pseudomonas aeruginosa ATCC9027, Escherichia coli ATCC8739 and Klebsiella pneumoniae ATCC1003 as Gram negative bacteria; Candida albicans ATCC1023 and Aspergillus niger ATCC16404 as fungi, which were obtained from Department of Drug and Food Control, Faculty of Pharmacy, Tehran University of Medical Sciences. Soybean Casein Digest Agar (Merck, Germany) and Saburouad Dextrose Agar (Merck, Germany) were used as medium for the growth of bacterial and fungal strains, respectively.
Antimicrobial assay
The antibacterial and antifungal activity of the different extracts and fractions of the plants were studied by cup plate diffusion method as described by Warnock DW (13). Each organism was separately suspended in normal saline solution which was equal to 108 CFU/mL. For preparing base plates, 25 mL of cooled media was poured in to the sterile Petri dishes and inoculated with one of the microorganisms by spreading microbial suspension over the plate with a sterile cotton swab. Then in each plate, holes of 7 mm in diameter were made at equal distances using sterile cork borer. Different concentrations of fractions (100, 50, 25, 12.5, 6.25, 3.125 and 1.562 mg/mL) were prepared and DMSO (dimethyl sulfoxide) with 1% w/v concentration was used as a solvent. 100 µL of each extracts and fractions were added to each hole on the medium. The plates containing bacteria and fungi were incubated at 35 ºC for 24 h and 25 ºC for 48 h, respectively. The diameter of zone of inhibition was measured in millimeters after incubation as an indication of activity and compared with the solvent as negative control. Gentamycin and Nystatin were used as positive control. All the tests were repeated in triplicate.
Elucidation of compounds of T. disciforme
Since there was few reports about phytochemical investigation of T. disciforme extract, it was selected for isolation and purification of compounds. Crude extract (313.61 g) from 1.5 Kg of T. disciforme was fractionated with petrol ether (PE) and chloroform (CH) yield 50.11 and 13.5 g respectively. Remaining gummy residue which was soluble in methanol called methanol fraction (ME; 250 g).
ME fraction (5 g) was applied to reverse phase silicagel column chromatography (2.5×13.5 cm) and eluted with gradient mobile phase H2O-MeOH (80:20 → 0: 100, V/V) to afford 5 subfractions. M3 subfraction (564 mg) was selected for size exclusion chromatography (SEC) on Sephadex LH-20 column (2.1×67 cm) eluted with MeOH. Compounds 1 (5.5 mg), 2 (4.3 mg) and 3 (12 mg) were isolated and purified. M4 subfraction (435 mg) subjected to SEC on Sephadex LH-20 column (2.1×67 cm) and MeOH: EtOAC (2:1) was used as solvent to obtain compound 4 (6.5 mg), 5 (3.8 mg) and 6 (9.5 mg). For further purification all compounds were applied to a Sephadex-LH20 CC (1.2×55 cm) eluted with methanol separately.
Spectral data of isolated compounds
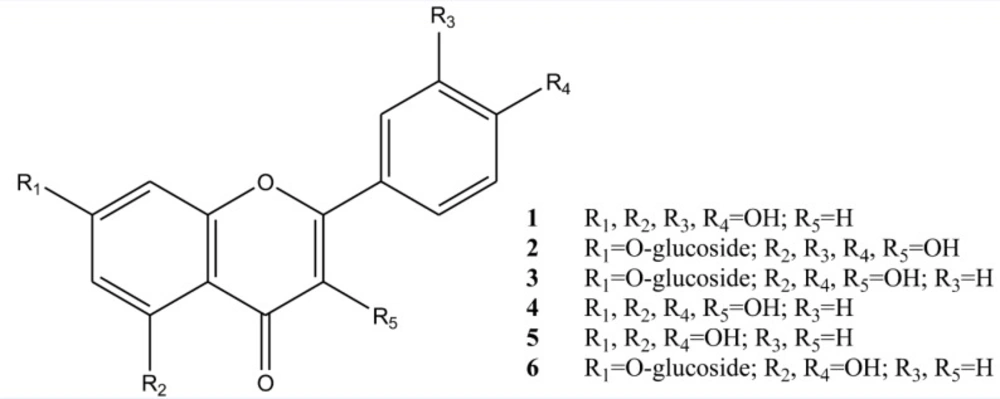
Luteolin 1: UV λ max nm MeOH: 345.5, 308, 284, 260sh; + AlCl3: 422, 307sh, 286; + AlCl3/HCl: 384, 350, 318sh, 307, 286; + NaOAC : 390, 307, 289 ; + NaOAC/H3BO3 : 430sh, 367, 370, 289; 1H NMR (400MHz, DMSO-d6): δ 7.46 (1H, dd, J=8.4, 2.0 Hz, H-6'), 7.00 (1H, d, J=8.4 Hz, H-5'), 7.52 (1H, d, J=2.0 Hz, H-2'), 6.57 (1H, s, H-3), 6.54 (1H, d, J=2 Hz, H-8), 6.25 (1H, d, J=2 Hz, H-6): 13C NMR(DMSO-d6): δ 182.9 (C-4), 165.2 (C-7), 165.4 (C-2), 162.3 (C-5), 158.5 (C-9), 150.1 (C-4'), 146.1 (C-3′), 122.8 (C-6′), 121.4 (C-1′), 115.9 (C-5'), 113.2 (C-2'), 102.9 (C-3), 102.9 (C-10), 99.2 (C-6), 94.1 (C-8).
Quercetin-7-O-glucoside 2: UV λ max nm MeOH: 369, 270sh, 250; + AlCl3: 441, 340sh, 270; + AlCl3/HCl: 430, 368sh, 292sh, 266; + NaOMe: 423, 270, 267sh, 246; + NaOAC : 256, 386 ; + NaOAC/H3BO3 : 254, 420; EIMS: m/z %: 302[M-glucose]+(100), 285 [M-OH]+(12), 273 [M-COH]+(8), 193 [M-B]+(12), 153[A1+H]+(27), 137[B2]+(32), 105[B1-COH]+(34); 1H NMR (400MHz, DMSO-d6): δ 7.56 (1H, dd, J=8.4, 2.4 Hz, H-6'), 7.74 (1H, d, J=2.4 Hz, H-2'), 6.91 (1H, d, J=8.4 Hz, H-5'), 6.77 (1H, d, J=2 Hz, H-8), 6.42 (1H, d, J=2 Hz, H-6), 5.08 (1H, d, J=7.6 Hz, H-1''), 3.5- 4.5 (5H, m, H-2''-6''); 13C NMR(DMSO-d6): δ 174.9 (C-4), 161.6 (C-7), 159.3 (C-5), 157.2 (C-9), 146.9 (C-4'), 146.5 (C-2), 144.0 (C-3'), 141.5 (C-3), 135.0 (C-1′), 120.7 (C-6′), 118.4 (C-2′), 114.5 (C-5'), 103.9 (C-10), 100.0 (C-1''), 98.8 (C-6), 98.0 (C-8), 76.1 (C-5''), 75.4 (C-3''), 72.1 (C-2''), 68.5 (C-4''), 59.6 (C-6'').
Kaempferol-7-O-glucoside 3: UV λ max nm MeOH: 367, 298sh, 267, 255; + AlCl3: 425, 345, 293sh, 266; + AlCl3/HCl: 425, 345, 293sh, 265; + NaOMe: dec; + NaOAC : 325sh, 380, 325sh, 266 ; + NaOAC/H3BO3 : 364, 256; 1H NMR (400MHz, DMSO-d6): δ 7.93 (2H, d, J=8.0 Hz, H-2',6'), 6.88 (2H, d, J=8.0 Hz, H-3',5'), 6.46 (1H, d, J=2.0 Hz, H-8), 6.20 (1H, d, J=2.0 Hz, H-6).
Kaempferol 4: UV λ max nm MeOH: 365, 320sh, 295sh, 266, 255; + AlCl3: 425, 330sh, 300sh, 272; + AlCl3/HCl: 425, 330sh, 300sh, 272;+ NaOMe: dec; + NaOAC : 390, 302, 268 ; + NaOAC/H3BO3 : 370, 320sh, 295sh, 265; 1H NMR (400MHz, DMSO-d6): δ 8.15 (2H, d, J=8.9 Hz, H-2',6'), 6.99 (2H, d, J=8.9 Hz, H-3',5'), 6.46 (1H, d, J=1.9 Hz, H-8), 6.27 (1H, d, J=1.9 Hz, H-6); 13C-NMR(DMSO-d6): δ 176.19 (C-4), 163.8 (C-7), 160.7 (C-5), 160.44 (C-4'), 156.75 (C-9), `149.0 (C-2), 136.19 (C-3), 129.52 (C-2′, 6′), 131.72 (C-1′), 115.45 (C-3', 5′), 102.56 (C-10), 98.05 (C-6), 93.5 (C-8).
Apigenin 5: UV λ max nm MeOH: 336, 284, 267.5; + AlCl3: 388, 345sh, 301, 276, 219; + AlCl3/HCl: 387, 343, 300, 276, 217; + NaOMe: 394, 318, 274, 214; + NaOAC : 359, 305, 272; + NaOAC/H3BO3 : 336, 268; EIMS: m/z %: 270[M-glucose]+(100), 152[A1](25), 121[B2](36), 118[B1](25); 1H NMR (400MHz, DMSO-d6): δ 7.76 (2H, d, J=8.4 Hz, H-2',6'), 6.95 (2H, d, J=8.4 Hz, H-3',5'), 6.51 (1H, s, H-3), 6.48 (1H, s, H-8), 6.24 (1H, s, H-6).
Apigenin-7-O-glucoside 6: UV λ max nm MeOH: 332, 268; + AlCl3: 385, 347, 299, 276; + AlCl3/HCl: 382, 341, 299, 277; + NaOMe: 386, 300, 279, 265; + NaOAC : 397sh, 341, 267; + NaOAC/H3BO3 : 336, 266, 256sh; EIMS: m/z %: 270[M-glucose]+(100), 152[A1](18), 120[B2-H](25), 117[B1-H](18); 1H NMR (400MHz, DMSO-d6): δ 7.84 (2H, d, J=8.0 Hz, H-2',6'), 6.94 (2H, d, J=8.0 Hz, H-3',5'), 6.72 (1H, s, H-3), 6.66 (1H, s, H-8), 6.44 (1H, s, H-3), 5.00 (1H, d, J=7.2 Hz, H-1''), 3.4- 4.5 (5H, m, H-2''-6'').
Results and Discussion
The antimicrobial effects of different fractions of S. securidaca seeds was demonstrated in Table 1. The Petroleum ether fraction only inhibited the growth of S. aureus and P. aeruginosa with inhibition zone diameter of 7.5 -12 mm. The chloroform fraction showed inhibitory effect only against S. aureus with inhibition zone of 9.5 -12 mm diameter. The methanol fraction showed no antimicrobial activity. The largest zones of inhibition were observed for petroleum ether fraction against P. aeruginosa and chloroform fraction against S. aureus (each 100 µg/mL). All fractions exhibited no antifungal activities.
| Inhibition zone diameter(mm) | Concentration | Sample | |||||
|---|---|---|---|---|---|---|---|
| CA | BS | KP | EC | PA | SA | ||
| - | - | - | - | 12 | 10 | 100 | Petroleum |
| - | - | - | - | 10 | 8 | 50 | |
| - | - | - | - | 9.5 | 7.5 | 25 | |
| - | - | - | - | - | - | 12.5 | |
| - | - | - | - | - | 12 | 100 | Chloroform |
| - | - | - | - | - | 10 | 50 | |
| - | - | - | - | - | 9.5 | 25 | |
| - | - | - | - | - | - | 12.5 | |
| - | - | - | - | - | - | 100 | Methanol Fraction |
The phytochemical analysis of S. securidaca showed existence of flavonoids, coumarins and cardiac glycosides (14-16). Some flavonoids of S. securidaca have been shown potent cytotoxicity by MTT assay against three Human cancer cell lines: colon carcinoma (HT-29), breast ductal carcinoma (T47D) and colorectal adenocarcinoma (Caco-2) (17).
There were reports for antimicrobial effects of some cardenolides (18, 19), and the antibacterial activity of S. securidaca may be due to existence of this class of compounds.
Rosa damascena extract showed good antibacterial activities against B. cereus, S. aureus, and S. epidermidis as Gram positive bacteria and P. aeruginosa as Gram negative bacteria with MICs (Minimum Inhibitory Concentration) 70, 140, 560 and 140 µg/mL, respectively. It was inactive against other microorganisms with MICs of >1000 μg/mL. The inhibition zone diameter of R. damascena extract against S. aureus and S. epidermidis is more than Gentamycin as positive control (5 µg/mL) (Table 2).
| Concentration | Inhibition zone diameter(mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| BC | BS | SA | SE | EC | PA | AN | CA | ||
| R. damascena Extract | 64 | 14 | - | 24 | 18 | - | 12 | - | - |
| 32 | 13.4 | - | 23 | 16 | - | 11 | - | - | |
| 16 | 13.3 | - | 18 | 15 | - | 10.3 | - | - | |
| 8 | 13.2 | - | 16 | 13 | - | 10 | - | - | |
| 4 | 13 | - | 13 | - | - | 9.6 | - | - | |
| 2 | 11 | - | 10 | - | - | 9 | - | - | |
| 1 | 10 | - | - | - | - | - | - | - | |
| 0.5 | - | - | - | - | - | - | - | - | |
| T. disciforme Extract | 64 | - | - | 14 | 12 | - | - | - | - |
| 32 | - | - | 10.2 | 10 | - | - | - | - | |
| 16 | - | - | 10 | - | - | - | - | - | |
| 8 | - | - | - | - | - | - | - | - | |
| 4 | - | - | - | - | - | - | - | - | |
| 2 | - | - | - | - | - | - | - | - | |
| 1 | - | - | - | - | - | - | - | - | |
| 0.5 | - | - | - | - | - | - | - | - | |
| Gentamycin | 5 | 25 | 18 | 18 | 12 | 18 | 19 | - | - |
| Nystatin | 50 | - | - | - | - | - | - | 23 | 25 |
A previous investigation showed the MIC of butanol extract of R. damascena receptacles against Salmonellatyphimurium and Bacillus cereus were 62.5 and 250 μg/mL, respectively.
Aqueous extract of R. damascena receptacles were inhibited Candida albicans and Methicillin-resistant S. aureus with MIC of 125 and 500 μg/mL (20). It is obvious that antimicrobial potential of crude extract of R. damascena against B.cereus was more than that of butanol extract. Another study demonstrated fresh and spent flower extracts of R. damascena showed the strongest effects against Salmonella enteritidis and Mycobacterium smegmatis. Both extracts were not effective against E. coli (9).
Tripleurospermum disciforme extract showed antimicrobial effects only against S. aureus and S. epidermidis with MICs 112 and 224 μg/mL, respectively. It was inactive against the other microorganisms (Table 2). Another study reported the essential oil of T. disciforme was effective on Staphylococcus subtilis and Bacillus cereus with MICs 4 µL/mL and on Citrobacter amalonaticus with MIC 22 µL/mL (21). Methanol extract of T. disciforme were not exhibited antiproliferative activity by using the MTT assay against: A549, human lung adenocarcinoma; MCF7, human breast adenocarcinoma; HepG2, hepatocellular carcinoma; HT-29, human colon carcinoma and one normal cell line MDBK, bovine kidney (22).
There was only one report about phytochemical investigation on flowers extract of T. disciforme which demonstrated isolation of a new dioxaspiran derivative (23). In our study, six flavonoids were isolated from T. disciforme: Luteolin, Quercetin-7-O-glucoside, Kaempferol, Kaempferol-7-O-glucoside, Apigenin and Apigenin-7-O-glucoside. The isolated compounds were identified using different spectroscopic methods (Figure 1).
Flavonoids act as antimicrobial agents in different ways including direct antibacterial activity, synergism with antibiotics and suppression virulence (24). Many researchers investigated the antibacterial activity of flavonoids (25), for example, it can be mentioned the antibacterial activities against Propionibacterium acnes by kaempferol and quercetin (26), inhibitory effects of apigenin against S. typhi, Proteus mirabilis and P. aeruginosa (27) and selective toxicity of apigenin and luteolin against S. aureus including the MRSA and methicillin-sensitive S. aureus strains (28, 29).
Conclusion
In conclusion, Rosa damascena and Tripleurospermum disciforme have shown antimicrobial effects against Staphylococcus strains. These results confirmed the folklore consumption of distilled water of R. damascena as tonic and face cleanser and fume of T. disciforme as tonic and disinfectant for treatment of acne. Because of antibiotic resistance of S. aureus, these two herbs can be used in health and beauty products for treatment of skin disorders especially acne in teenagers.