Introduction
Malaria continues to be a life threatening disease in the tropical and subtropical regions with the strongest mortality (1). It is transmitted by protozoa of the genus plasmodium and responsible for hundreds of millions of infections that kill between one and three million people annually (2). This situation has been complicated by the emergence of parasite strains resistant to the existing inexpensive drugs such as chloroquine (3); therefore, there is an urgent need to find alternative drugs especially traditional and herbal remedies for the treatment of the disease. Members of the genus Artemisia (Asteraceae) are important medicinal plants, with about 400 species wildly distributed in the northern hemisphere (especially in Europe, North America, Asia and South Africa) and represented in Iranian flora by 34 species (4, 5). This genus has been gaining increasing attention since the discovery of artemisinin, a promising and potent antimalarial drug which derived from the plant A. annua (6). Experiments suggested that artemisinin and its derivatives kill plasmodium protozoa by interacting with heme to produce free radicals that alkylate specific malarial proteins and damage membranes of the parasite. Moreover, artemisinin could inhibit heme bio crystallization and interact with hemozoin formation, lead to split of the malaria pigment (7, 8). Recently, the DCM extracts of A. scoparia and A. spicigera were shown to significantly inhibit the heme bio crystallization in β-hematin formation assay (9). In continuation of our studies on Iranian Artemisia species, we have now evaluated antimalarial effect of different extracts from three Artemisia species including A. ciniformis, A. biennis and A. turanica. Recently, the total extract of A. turanica was reported to have antimalarial effect against Plasmodium berghei (10). In other studies, ethanol extract of A. turanica has shown anticancer activity against human Caucasian hepatocyte carcinoma (HepG-2) and human Caucasian larynx carcinoma (Hep-2) cell lines (11). Moreover, methanol extract of this plant was reported to have antimicrobial activity (12). DCM extracts of A. biennis and A. ciniformis have been shown to inhibit cancer cell growth (13), likewise, different extracts of A. ciniformis have been reported to possess antiprolifrative effects on malignant cell lines (14, 15). It was recently reported that the ethanol extracts of these three species have inhibitory effects against Leishmania major parasites (16) and the hydroethanolic extract of A. biennis showed potent antioxidant activity in different assays (17). In the current study, the anti-malarial activity of different extracts from these three Artemisia species was examined by in-vitroβ-hematin formation assay.
Experimental
Chemicals
Hematin procine, chloroquine diphosphate, sodium dodecyle sulfate (SDS), sodium acetate, magnesium sulfate, sodium hydrogen phosphate, sodium chloride, potassium chloride, sodium hydroxide, glucose and sodium bicarbonate were purchased from Sigma-Aldrich Chemical Company, oleic acid from Fluka, dimethyl sulfoxide and hydrochloric acid from Merck and all the solvents used for extraction from Caledon and Scharlau.
Plant material
The aerial parts of A. ciniformis Krasch. & M. Pop. Ex Poljak, A. biennis Willd. and A. turanica Krasch. were collected from Tandoreh National park, Zoshkand Sami abad, Torbat- e Jam (Razavi Khorasan province, Iran) respectively. Samples were identified by Dr Valiollah Mozaffarian (Research Institute of Forest and Rangelands, Tehran, Iran). The voucher specimens (Nos. 12569, 12570 and 12572, respectively) have been deposited in the herbarium, Department of Pharmacognosy, Faculty of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran.
Extract Preparation
The plant materials were air-dried at room temperature, finely ground and extracted by maceration method (18). 100 g of each plant was extracted successively with petroleum ether (PE), DCM, EtOAC, ethanol and ethanol-water (1:1 v/v) at room temperature (Sequential maceration with ca. 3×1 L of each solvent). All the extracts were separately concentrated using a rotary evaporator at a maximum temperature of 45 °C.
In-vitro β-hematin formation assay
The antimalarial activity of plant extracts was evaluated by the in-vitroβ-hematin formation assay described by Afshar et al. (9) with some modifications. Briefly, varying concentrations (0.4- 2 mg/mL in DMSO) of each extract were mixed with 3 mM of hematin, 10 mM oleic acid and 1 M HCl. The final volume was adjusted to 1 mL using sodium acetate buffer, pH 5. Chloroquine diphosphate was used as a positive control. The reaction mixtures were incubated overnight at 37 °C with constant gentle shaking. Incubation was terminated by centrifugation (14000 rpm, 10 min, at 21 °C) to collect the β-hematin pellets. The pellets repeatedly washed with incubation (15 min at 37 °C with regular shaking) in 2.5% (w/v) SDS in phosphate buffer saline followed by a final wash in 0.1 M sodium bicarbonate, until the supernatant was colorless. To determine the heme amount crystallized into β-hematin, the pellets were dissolved in 0.1 M NaOH and measured the absorbance at 400 nm (Beckman DU640 spectrophotometer). The results were recorded as % inhibition (I%) of heme crystallization compared to negative control (DMSO) using the following equation: I% = [(AN–AS)/AN]*100, where AN: absorbance of negative control; AS: absorbance of test samples.
Statistical analyses
All experiments were conducted in triplicate measurements and presented as the mean ± standard deviation. Data were analyzed by using SPSS, version 16.0.0 software. The IC50 and IC90values were calculated from non-linear regression analysis.
Results and Discussion
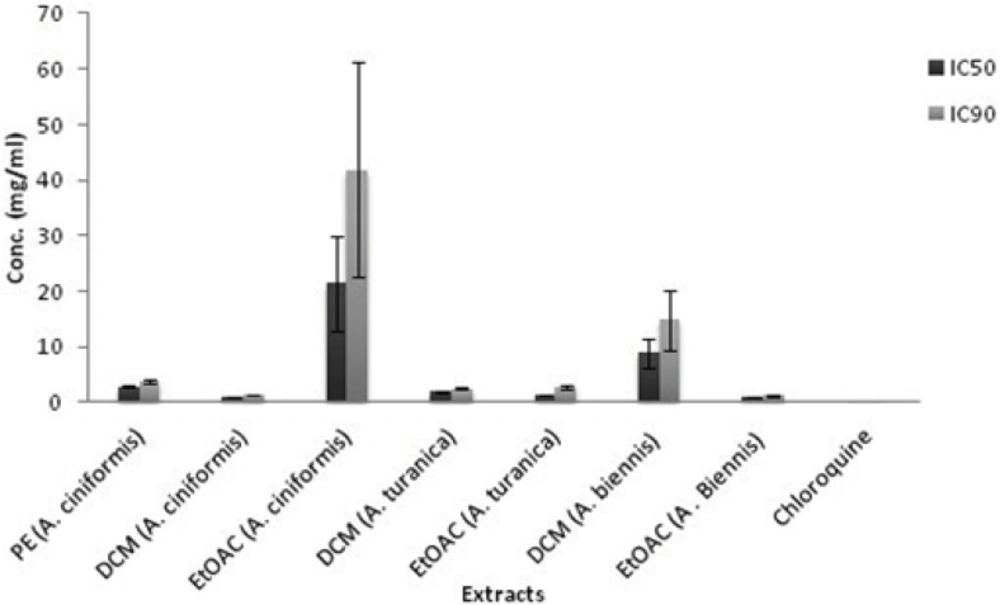
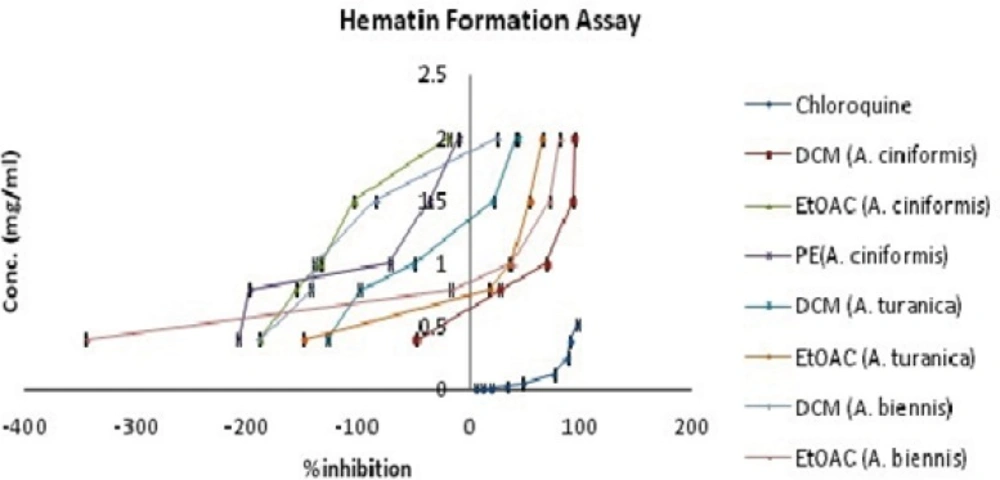
During the intra-erythrocytic cycle, the malaria parasite digests the host hemoglobin within the food vacuoles of infected erythrocytes as the main source of nutrition for its development and maturation (19, 20). Massive degradation of hemoglobin is accompanied by the release of toxic free heme which affects cellular metabolism and causes parasite death (21, 22). To get rid of the excess heme, the malaria parasites have evolved a detoxification pathway which converted heme into an inert and insoluble crystal known as hemozoin or malaria pigment (23). Hemozoin bio crystallization is an essential process for the malaria parasite and is a validated target for antimalarial chemotherapy as well as drug screening programs (24). Several in-vitro bioassays based on differential solubility and spectral characteristics of monomeric heme and β -hematin (synthetic analogue of hemozoin) have been defined and exerted for searching of novel synthetic and natural antimalarial compounds (19, 24, 25). In the present investigation, the antimalarial activity was evaluated by the in-vitroβ-hematin formation assay developed by Afshar et al. (9). The results from the antimalarial testing of fifteen extracts of A. ciniformis, A. turanica and A. biennis as well as the extraction yields are presented in Table 1. The IC50 and IC90 values for each active extract were calculated graphically by plotting concentrations against percentage of inhibition (I%) and defined as the concentration of extract causing 50% and 90% inhibition of β-hematin formation, respectively. As illustrated in Table 1 and Figure 1, ethanol, ethanol-water and PE extracts revealed no activities in this assay system except for PE extract of A. ciniformis (IC50 = 2.88 ± 0.26 mg/mL, IC90 = 3.86 ± 0.40 mg/mL), while the DCM extracts of A. ciniformis and A. turanica as well as EtOAC extracts of A. biennis and A. turanica were found to be the inhibitors of β-hematin formation. The most potent antimalarial activities belonged to DCM extract of A. ciniformis (IC50 = 0.92 ± 0.01 mg/mL, IC90 = 1.29 ± 0.02 mg/mL), followed by EtOAC extracts of A. biennis (IC50 = 1.11 ± 0.02 mg/mL, IC90 = 1.22 ± 0.04 mg/mL) and A. turanica (IC50 = 1.35 ± 0.08 mg/mL, IC90 = 2.81 ± 0.21 mg/mL).Using box and whisker plots for IC50 and IC90 values revealed the presence of an outlier that was related to EtOAC extract of A. ciniformis. In other words, the rest of active samples could be remained as candidates for further study and comparison. Chloroquine was tested as a reference drug with IC50value of 0.04 ± 0.01 mg/mL and IC90 value of 0.35 ± 0.01 mg/mL. It was demonstrated that compounds with potent antimalarial activity in these active extracts have medium polarity. Previous researches on natural compounds showed that terpenes, steroids (26), saponins (27), methoxylated flavonoids (28) and methylated coumarins (29) exhibited antimalarial effects in various tests. Also, according to the screening study on terpenoid content of ten Iranian Artemisia species carried out by Iranshahi et al. (30), A. cinifomis showed high content of sesquiterpenoid lactons while A. biennis and A. turanica have low amount of terpenes. Therefore, it seems that the potent antimalarial activity of DCM extract from A. ciniformis might be due to the high content of sesquiterpenoid lactones. In the case of A. turanica and A. biennis, the antimalarial activity of EtOAC extracts was superior to the corresponding DCM extracts. These results might have been derived from the high concentration of antimalarial component with higher polarity than sesquiterpenoids like methoxylated flavonoids or methylated coumarins and removing as much the lipid like compounds from these extracts. As represented in Figure 2, at lower concentrations of the potent extracts and at all concentrations (0.4-2 mg/mL) of weak extracts (PE and EtOAC extracts of A. ciniformis), the percent inhibition values were negative, because the observed absorbences were higher than the negative control. These data are in agreement with our previous study (9) that showed that the presence of lipids and other fatty acids in the mixture of semi-polar extracts cause synergistic effect with oleic acid in the assay. It was indicated that the IC50 and IC90 values could be decreased by entirely removing the lipids and purification of the active antimalarial compounds.
| IC90 (mg/mL)a | IC50 (mg/mL)a | Yields (%) | Extracts/Fractions | Plants |
|---|---|---|---|---|
| 3.86 ± 0.40 | 2.88 ± 0.26 | 5.31 | petroleum ether | A. ciniformis |
| 1.29 ± 0.02 | 0.92 ± 0.01 | 11.58 | dichloromethane | |
| 41.85 ± 19.28 | 21.46 ± 8.44 | 0.42 | ethyl acetate | |
| - | - | 3.28 | ethanol | |
| - | - | 20.72 | ethanol-water | |
| - | - | 2.74 | petroleum ether | A. turanica |
| 2.49 ± 0.17 | 1.93 ± 0.09 | 12.11 | dichloromethane | |
| 2.81 ± 0.21 | 1.35 ± 0.08 | 0.60 | ethyl acetate | |
| - | - | 3.85 | ethanol | |
| - | - | 18.69 | ethanol-water | |
| - | - | 5.27 | petroleum ether | A. biennis |
| 14.80 ± 5.50 | 9.02 ± 2.64 | 7.22 | dichloromethane | |
| 1.22 ± 0.04 | 1.11 ± 0.02 | 0.46 | ethyl acetate | |
| - | - | 1.42 | ethanol | |
| - | - | 9.94 | ethanol-water | |
| 0.35 ± 0.01 | 0.04 ± 0.01 | - | - | Chloroquine |
Experiment was performed in triplicate and the results were expressed as Mean ± SD.
Conclusion
The plant extracts in this investigation are less active antimalarials than the reference drug, chloroquine, but these extracts contain a heterogeneous mixture of various compounds and the active components might display more potent activity in their pure form. Among fifteen tested extracts, the DCM extract of A. ciniformis was considered more promising for further studies to isolate and identificate the active antimalarial principles.