Introduction
Platelets play an important role in maintaining cardiovascular integrity and in regulating the bleeding process by blood-clot formation (1). However, uncontrolled platelet aggregation is dangerous in arterial blockage and may lead to life threatening disorders (2). Antiplatelet agents are therefore considered as a significant tool in the treatment and/or prevention of cardiovascular thrombotic disease (3-5). Antiplatelet agents such as aspirin (acetylsalicylic acid), clopidogrel or ticlopidine and anticoagulants such as warfarin are currently two predominant groups of orally consumable drugs in standard therapeutic protocols for prophylaxis and treatment of venous thrombosis and reducing the risk of recurrent myocardial infarction (6-8).
Currently aspirin, which irreversibly inhibits cyclooxygenase I-mediated transformation of arachidonic acid to thromboxane A2 (TXA2), and the P2Y12 antagonists clopidogrel and prasugrel, which selectively and irreversibly bind to theP2Y12 ADP receptor are routinely used as antiplatelet agents (9, 10).
However there are still some serious limitations to these agents which include weak inhibition of platelet function (eg. aspirin) (11), slow onset of action (eg. clopidogrel) (12), variable response to treatment among the patients (eg. clopidogrel and aspirin) (11, 12) and high incidence of bleeding events which occur in both aspirin and clopidogrel drug therapy (13, 14). Considering the current situation, development of novel antiplatelet agents which are safe and effective is an urgent need (15).
Aminopyrimidine derivatives are an interesting group of compounds with various reported biological properties. Pyrimidine ring can be found in the structures of many important drugs such as nucleoside antibiotics, antibacterials and cardiovascular agents (16, 17).
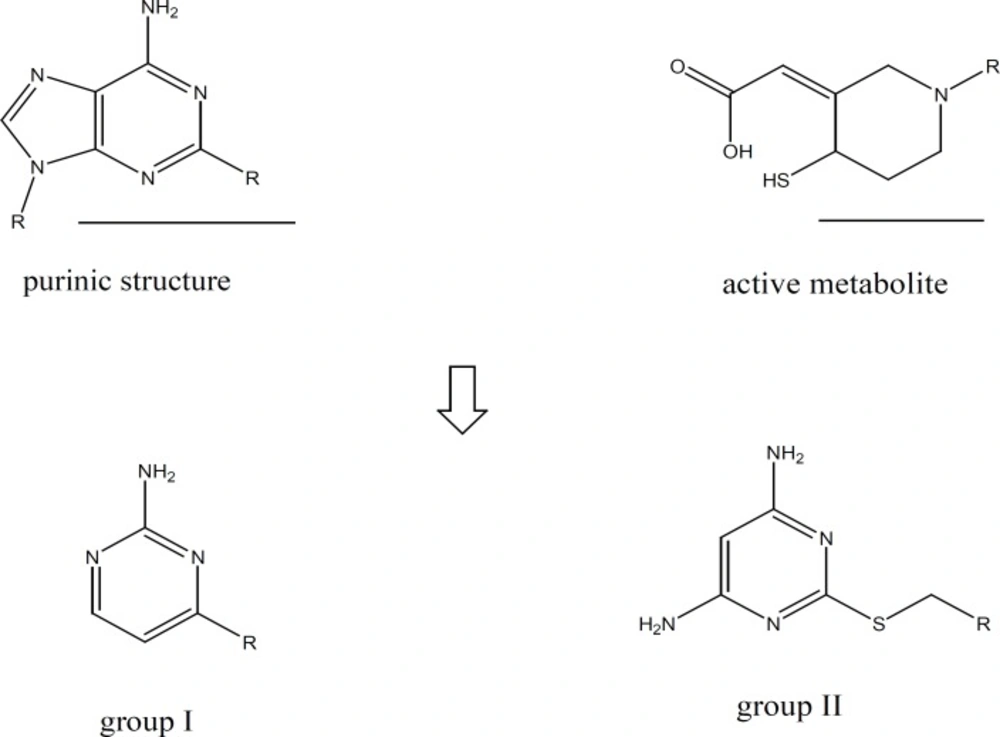
Based on the hypothesis suggested by Cattaneo et al. (18), amino pyrimidine ring could be considered as a simplified form of the active metabolites of the thienopyridines and ATP derivatives. The active metabolites of thienopyridines have a simple monocyclic structure which implies that the presence of a bicyclic structure like that of a purine ring is not an absolute requirement for the affinity for the ADP receptor or so on platelet membrane.
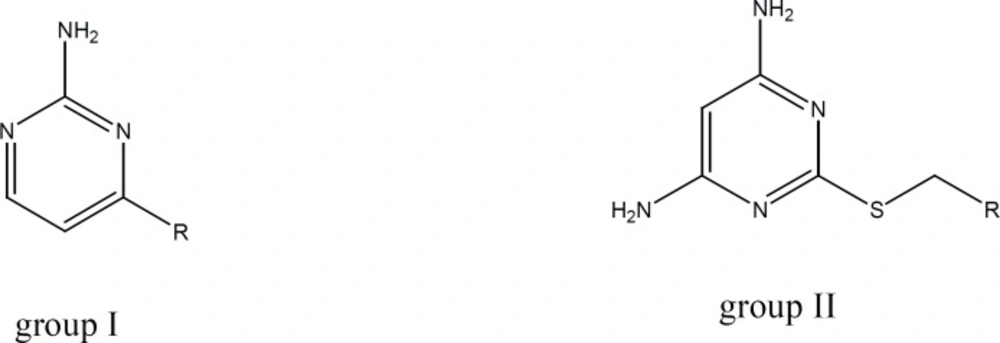
A group of amino pyrimidine derivative with thioether substituents has been synthesized and evaluated by Cattaneo et al. The compound showed satisfactory anti platelet aggregation effects when ADP had been used for aggregation induction (18). Based on the mentioned reports and in order to investigate the capability of amino pyrimidine derivatives in inhibition of platelet aggregation pathways, we synthesized two groups of amino pyrimidines with the following structures:
Comparing the activity of these compounds in inhibition of platelet aggregation induced by ADP and arachidonic acid will provide some insights into the structure activity relationship of these compounds.
Chemistry
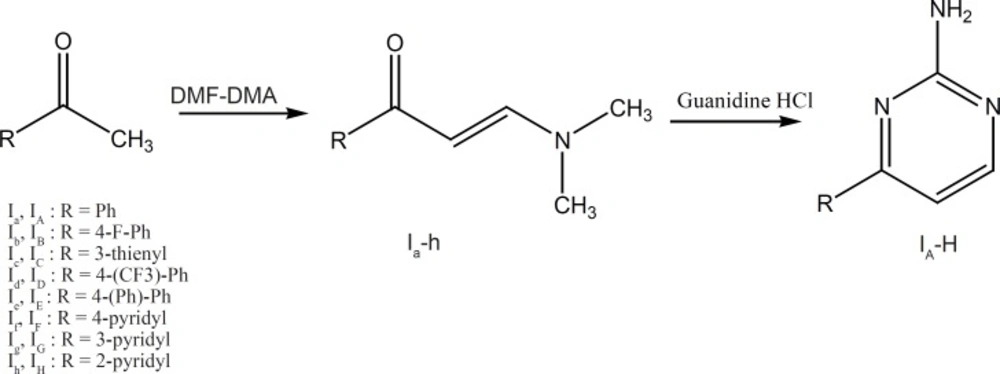
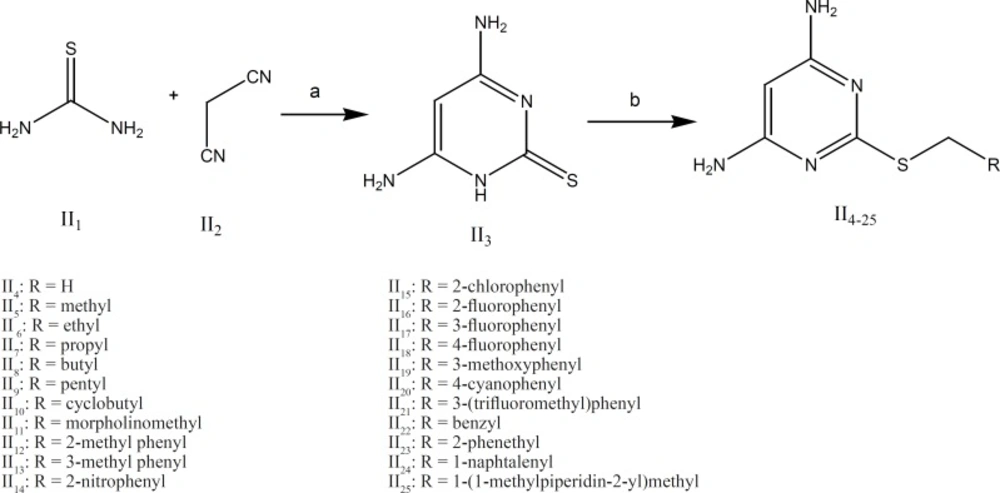
The synthetic procedures for groups I and II are illustrated in Figures 4 and 5.
Group I: Methyl ketones (1a-h) were allowed to react with dimethylformamide-dimethylacetate (DMF-DMA) to produce 3-(dimethylamino)-1-aryl-2en-1-ones (2a-h). These intermediates could be then condensed with guanidine HCl to obtain the corresponding amino pyrimidine ring systems (19).
Group II: As it is shown in Figure 5 the intermediate II3 (4,6-diaminopyrimidine-2-thiol) was obtained by the reaction of thiourea and malononitrile in absolute ethanol as the solvent. Subsequent reaction of compound II3with various alkylhalides at room temperature afforded compounds II4-25 in good yields.
Structure confirmation of the synthesized intermediates and the final products was performed using IR, NMR and Mass spectrometry (20).
Experimental
General
All evaporations were carried out in vacuo with a rotary evaporator. Melting points (˚C) were determined by capillary method on an electrothermal melting point apparatus. Infrared spectra were recorded as thin films of KBr plates with Umax in inverse centimeters. Nuclear magnetic resonance spectra for proton (1H NMR) were recorded on a Bruker DRX-Avance (500 MHz) spectrometer. Chemical shift values are expressed in ppm (parts per million) relative to tetramethylsilane (TMS) as internal standard; s: singlet, d: doublet, dd: double doublet, t: triplet, q: quartet, m: multiplet, br s: broad singlet. Thin layer chromatography (TLC) was performed on Whatman SilG/UV254 silica gel plates with fluorescent indicator and the spots were visualized under 254 and 366 nm illumination. Mass analyses were performed on an Agilent 6400 series equipped with an electrospray (ESI) ionization interface (drying gas adjusted at 300 ˚C, nebulizing gas flow at 12 L/min). All the compounds were analyzed for C, H, N and S on a Costech model 4010 and agreed with the proposed structures within ±0.4% of the theoretical values.
General procedure for the preparation of compounds (Ia-h)
The detailed description of the methods used for preparation of compounds Iato Ih and compounds IA to IH is reported in reference 19. Briefly to a solution of acetophenone (84 mmol) in DMF (16 mL), was added DMF-DMA (84 mmol) and the solution was heated under reflux for 24 h. Brine (25 mL) was added to the reaction mixture after cooling and the reaction mixture was then extracted with CH2Cl2 (3 × 25 mL). The combined organic fractions were dried over anhydrous Na2SO4 and the solvent was evaporated under reduced pressure. The residue was dissolved in EtOAc (12 mL), followed by addition of n-hexane (100 mL). The precipitate thus obtained was filtered and dried to give the pure product Ia as a yellow solid (19).
General procedure for the preparation of compounds (IA-H)
To a solution of compounds Ia (2.68 mmol) in isopropanol (13.5 mL) were added sodium methoxide (10.7 mmol) and guanidine hydrochloride (4.02 mmol) and the solution was heated under reflux for 48h. Distilled water (25 mL) was added to the reaction mixture after cooling and the mixture was then extracted with EtOAc (3 × 15 mL). The combined organic fractions were dried over anhydrous Na2SO4 and the solvent was evaporated under reduced pressure. The residue was dissolved in EtOAc (2 mL), followed by addition of n-hexane (25 mL). The precipitate thus obtained was filtered and dried to give IA-H.
Representative procedure for the preparation of compounds (II4-25)
The detailed description of the methods used for preparation of compounds II4 to II25is reported in reference 20. Briefly, to a solution of 4,6-diaminopyrimidine-2-thiol (II3, 1.4 mmol) in methanol, was added alkyl halide (3.5 mmol) under basic conditions (NaOH 0.1 M, 14 mL). The mixture was then stirred for 18h at room temperature. After removing the solvent under reduced pressure, the residue was washed by water and the precipitate was collected as a solid. All compounds were obtained in acceptable purity and no further purification was needed (20).
Results and Discussion
All the synthesized compounds were screened for their effects on human platelet aggregation induced by arachidonic acid and ADP using light transmission aggregometry. IC50 was determined as the concentration of the test compounds that exhibit platelet aggregation by 50%. The in-vitro antiplatelet activity of the synthesized derivatives is listed in Tables 1 and 2.
| Compound | R | A.A IC50 | ADP |
|---|---|---|---|
| Ia (1.25 mM) | ph | ||
| IA (0.75 mM) | ph | ||
| Ib (1 mM) | 4-F- ph | ||
| IB (2.5 mM) | 4-F- ph | ||
| Ic (1 mM) | 3-thienyl | ||
| IC (1.6 mM) | 3-thienyl | ||
| Id (1.3 mM) | 4-(CF3)- ph | ||
| ID (0.5 mM) | 4-(CF3)- ph | ||
| Ie (1.3 mM) | 4-( ph)- ph | ||
| IE (1 mM) | 4-( ph)- ph | ||
| If (1 mM) | 4-pyridyl | ||
| IF (1 mM) | 4-pyridyl | ||
| Ig (2.5 mM) | 3-pyridyl | ||
| IG (0.5 mM) | 3-pyridyl | ||
| Ih (1.25 mM) | 2-pyridyl | ||
| IH (1.25 mM) | 2-pyridyl | ||
| Indomethacin | |||
| Aspirin |
Comparing the activities of the two aminopyrimidine groups indicates that none of the compounds showed satisfactory activity against the aggregation induced by ADP. Therefore it could be concluded that the compounds do not interfere with ADP receptors on platelet membrane. Howeveracceptable activities were observed in both groups against the aggregation induced by arachidonic acid. This is not in agreement with the report by Cattaneo et al. who introduced a group of aminopyrimidines as active platelet aggregation inhibitors which interfere with ADP receptors
| Compound | R | A.A IC50 | ADP % inhibition |
|---|---|---|---|
| II4 | H | ||
| II5 | Methyl | ||
| II6 | Ethyl | ||
| II7 | Propyl | ||
| II8 | Butyl | ||
| II9 | Pentyl | ||
| II10 | Cyclobutyl | ||
| II11 | Morpholinomethyl | ||
| II12 | 2-methylphenyl | ||
| II13 | 3-methylphenyl | ||
| II14 | 2-nitrophenyl | ||
| II15 | 2-chlorophenyl | ||
| II16 | 2-fluorophenyl | 80 | |
| II17 | 3-fluorophenyl | ||
| II18 | 4-fluorophenyl | ||
| II19 | 3-methoxyphenyl | ||
| II20 | 4-cyanophenyl | ||
| II21 | 3-(trifluoromethyl)phenyl | ||
| II22 | Benzyl | ||
| II23 | 2-phenethyl | ||
| II24 | 1-naphthalenyl | ||
| II25 | 1-(1-methylpiperidin-2-yl)methyl | ||
| Indomethacin | |||
| aspirin |
Comparing the overall results obtained for aminopyrimidines I and II indicates that 2-aminopyrimidines (I) were more active than 4,6-diaminopyrimidines (II). Only compound 16 in group II showed satisfactory IC50 (80 µM).
Among the 2-aminopyrimidines group (I), on the other hand, a few compounds (Ia, Ib, IB and IG) showed good activities (36.75, 72.4, 62.5 and 192 µM)Interestingly, compounds with fluorine substituent on phenyl ring (Ib, IB) were among the most active compounds.
Global physicochemical properties for compounds Ia-h, IA-H and II4-25 were calculated using Chemdraw Ultra, Chem3D Ultra version 8.0 and Hyper Chem professional and the results are presented in Tables 3 and 4.
Efforts to find a relationship between these physicochemical parameters and anti platelet aggregation activity of the compounds did not result in a clear correlation. This could be due to the fact that the compounds are different in their access to their targets in the platelet aggregation pathway induced by arachidonic acid.
Further mechanistic studies are needed to clarify the mechanism of antiplatelet activity of these compounds.
ClogP were calculated by using Chem Draw Ultra version 8.0.
Polarizability values were calculated by using Hyper Chem Professional.
Molecular volume values were calculated by using Hyper Chem Professional.
Surface area values were calculated by using Hyper Chem Professional.
Dipole (debye) values were calculated by using Chem3D Ultra version 8.0.
ClogP were calculated by using Chem Draw Ultra version 8.0.
Polarizability values were calculated by using Hyper Chem Professional.
Molecular volume values were calculated by using Hyper Chem Professional.
Surface area values were calculated by using Hyper Chem Professional.
Dipole (debye) values were calculated by using Chem3D Ultra version 8.0.
![Active antiplatelet pyrimidine derivatives. A. 6-alkylamino-2,4-dialkyl(aryl)thiopyrimidines [7], R = Me,](https://services.brieflands.com/cdn/serve/3170b/38ef35c1606eb95b02fa6f3890ecaf617dc80184/ijpr-14-417-g001-preview.webp)