Introduction
Umbelliferae family contains about 300 genera and 2500-3000 species distributed all around the world (1). The genus Pimpinella is one of the main genera of Umbelliferae and comprises more than 150 species (2). The genus represents in the flora of Iran by twenty species including six endemics (3). Previous phytochemical studies of Pimpinella species have led to the isolation of various compounds like phenylpropanoids (4) sesquiterpenes (5) coumarins (6) and volatile oils (7). According to pharmacological studies, the fruit of Anis (Pimpinella anisum) is widely used as carminative, expectorant and spasmolytic (8). It is also proved to possess antioxidant, antimicrobial, gastroprotective, antifungal, anticancer and bronchodilatory activities (9-14). It is used in traditional medicine for menopausal hot flashes (14).In-vitro study of P. brachycarpa, edible greens grown in Asian regions, is found to have antioxidant effects (15). P. anisoides inhibits acetylcholinesterase (16) and presents protective effect on oxidative damages (17). P. tirupatiensis have also shown cardio-protective activity on doxorubicin induced cardiotoxicity in rats (18). Economically, this genera are cultivated all around the world as medicinal plant. A few other species are cultivated for their aromatic fruits such as P. anisetum in Russia and P. saxifraga in India. P. peregrina and P. major are cultivated in Germany for their roots and P. calycina as vegetable (18). Pimpinella haussknechtii Rech. f. & Riedl (Syn. P.kotschyana Boiss.) (20) is an annual native plant which grows in the west of Iran.
Available information indicates that flavonoids and essential oils are two secondary metabolites which have been reported from different parts of P.kotschyana (22, 21).
There are also a report on chemical composition and antimicrobial activity of P.kotschyana oil collected from Tehran province, Iran (23).
In this study, the volatile oil constituents of the friuts of P. haussknechtii grown in Lorestan province, Iran is reported by using the GC-MS analysis for the known components and high pressure liquid chromatography for unknown compound.
Experimental
General
HPLC (High-performance liquid chromatographic) analysis was done on a Waters system, equipped with 515 HPLC pump, and waters 2487 dual wavelenghth absorbance detector (Waters, Milford, MA, USA). The column was a YMC-Pak SIL (250 × 20 mm) (YMC Europe GmbH, Germany). The NMR spectra were recorded on a Bruker Avance AV 400 instrument, using CDCl3 as a solvent. The IR spectrum was recorded on a Rayleigh WQF-510 FTIR spectrophotometer and the HREI-MS spectrum was measured in electron impact mode on Varian MAT 312 spectrometer.
Plant material
The fruits of P. haussknechtii were collected during July 2012 from Khoramabad in the west of Iran at an altitude of ca. 1100 m above sea level. The plant was identified by Khoramabad Agricultural and Natural Resource Research Center. A voucher specimen (No 2827) was deposited at the Herbarium of the School of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences, Isfahan, Iran.
Extraction and isolation
The essential oil of the fruits of P. haussknechtii was obtained by hydrodistillation using a Clevenger-type apparatus for 3 h according to the method recommended by the British Pharmacopoeia (24). The volatile oil was dried over anhydrous sodium sulfate and stored in sealed vial at 4°C until analysis. Gas chromatography combined with mass spectrometry was used for identification of the known oil components. Firstly, the analysis was performed on an Agilent 5975C mass selective detector coupled with an Agilent 7890A GC, equipped with an HP-5 GC capillary column (30 m × 0.25 mm; film thickness 0.25 μm). The oven temperature was programmed from 60-280°C at the rate of 4°C per min. Helium was used as the carrier gas at a flow rate of 2 mL/min. Injector and detector temperatures were 280°C. The MS operating parameters were: ionization voltage, 70 eV; ion source temperature, 230°C; mass range, 35-425. The MSD ChemStation was used as operating software. Retention indices were calculated by using retention times of n-alkanes (C8-C24) that were injected after the oil at the same conditions. Components of the oil were identified by comparison of their retention indices (RI) with those reported in the literature (21) and computer matching with NIST and Wiley275. L libraries. The fragmentation patterns of the mass spectra were also compared with those reported in the literature (25, 27).
After GC-MS analysis, one unknown component (56.7%) was observed with retention time of 22.8 min, not characterized in the GC-MS library. In order to identify this compound, the essential oil was subjected on HPLC using YMC-Pak-Sil column (250 × 20 mm) with gradient system of hexane (A), and hexane: ethyl acetate, 9:1 (B) starting with A: B (100: 0) for 20 min, then 0−20% B in 50 min, A:B (80: 20) for 50 min, then 20-30% B in 30 min, and 30-100% B for 50 min. The flow rate was 3 mL/min, UV ditection at 210 and 270 nm , and the injection volume was 100 μL. The composition of each fraction was controlled by GC/MS analysis and the HPLC retention time for the compound of interest was found to be 116-122 min.
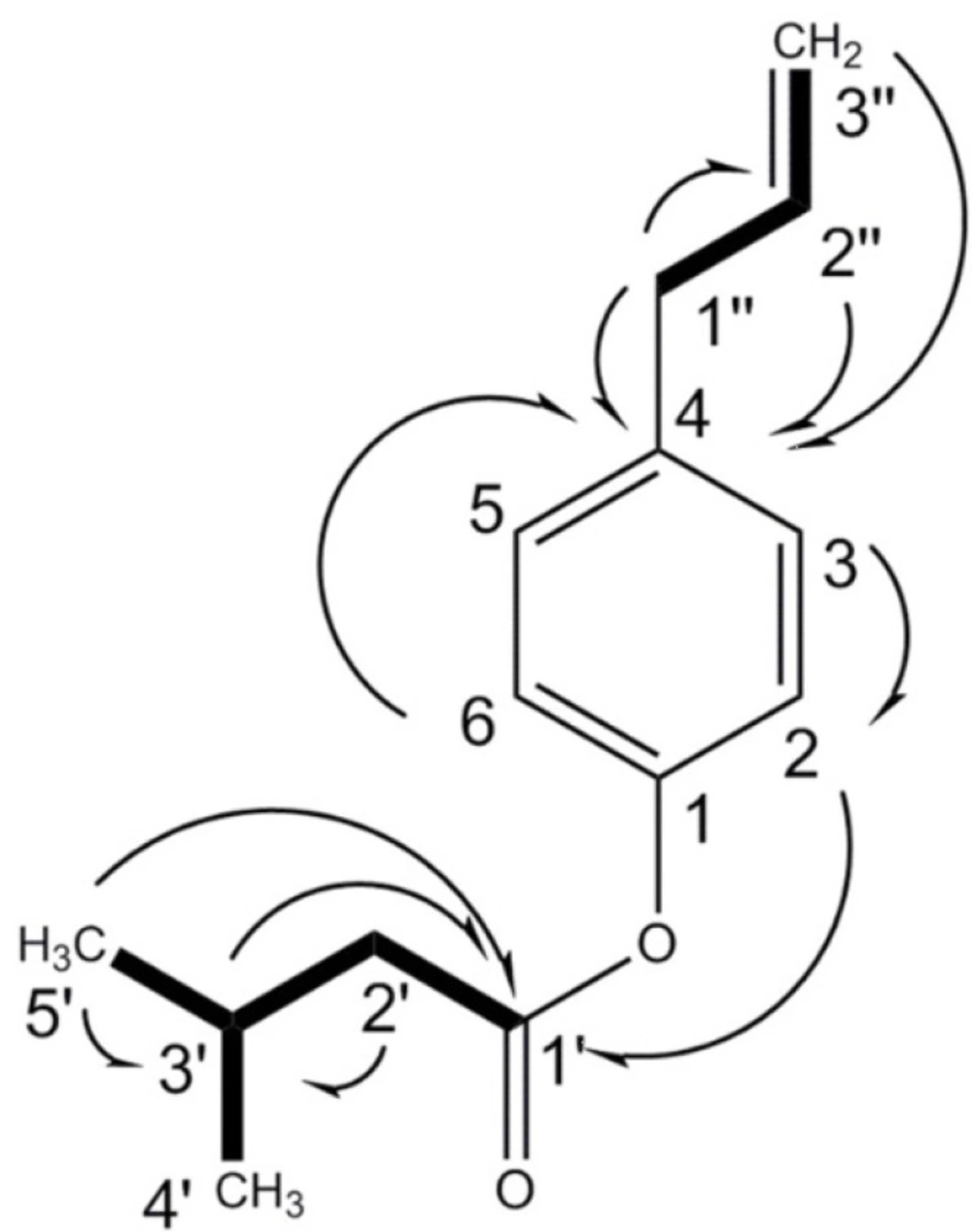
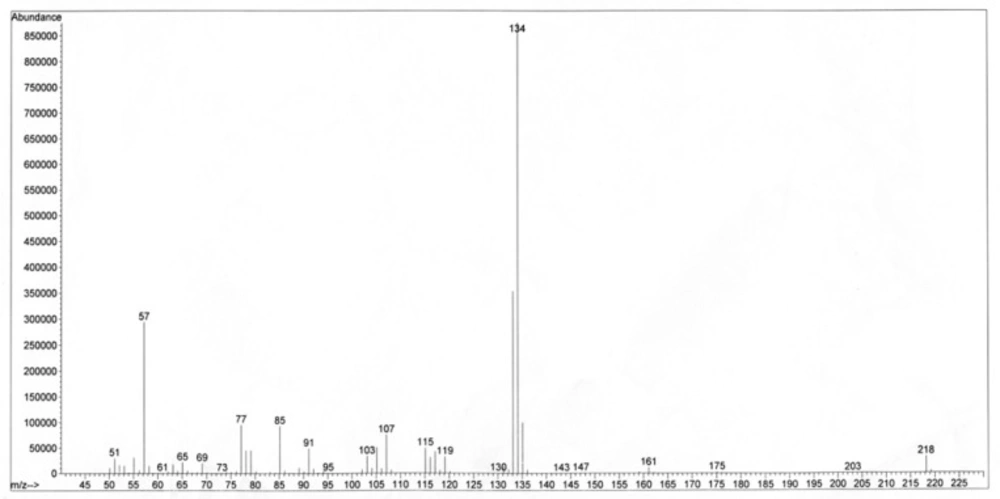
4-(prop-2-enyl)-phenyl-3'-methylbutanoate (1). White solid; [α]D: −20.4 (c 0.18, CDCl3); IR (KBr) νmax:3080, 3005, 2962, 2933, 2873, 1759, 1639, 1608, 1506, 1468, 1435, 1417, 1369, 1292, 1203, 1165, 1101, 1018, 995, 916, 850, 771 cm-1; 1H-NMR (CDCl3, 400 MHz, J in Hz) and 13C-NMR (CDCl3, 100 MHz) see Table 1. HREI-MS m/z 218.1296 (calc. for C14H18O2, 218.1307, Δ -4.8 ppm), Positive EI-MS m/z 218 (10), 134 (100), 119 (12), 115 (15), 107 (20), 105 (11), 103 (7) , 91 (16), 85 (22), 77 (23), 57 (40).
| Pos | δH, mult., J in Hz | δC | Pos | δH, mult., J in Hz | δC |
|---|---|---|---|---|---|
| 1 | - | 171.7 | O-3'-MB | ||
| 2 | 6.91 d (8.4) | 121.5 | 1' | - | 171.7 |
| 3 | 7.12 d (8.4) | 129.5 | 2' | 2.35, d (7.2) | 43.4 |
| 4 | - | 140.4 | 3' | 2.18, m | 25.9 |
| 5 | 7.12 d (8.4) | 137.5 | 4' | 0.98, d (6.8) | 22.4 |
| 6 | 6.91 d (8.4) | 121.5 | 5' | 0.98, d (6.8) | 22.4 |
| 1'' | 3.30, d (6.4) | 39.6 | |||
| 2'' | 5.88, m | 137.2 | |||
| 3''a | 5.03, dd (18.4, 1.8) | 116.0 | |||
| 3''b | 5.01, dd (10.8, 1.8) |
Results and Discussion
More than thirty-six components were detected in the fruits of P. haussknechtii (Table 2). Thirty-five components of the oil were identified by GC-MS method and then HPLC method was used for the isolation of one unknown component (56.7%), which was not characterized in the GC-MS library with retention time of 22.8 min.
| No | Compound | RT | RI Calc.a | RIb | %c | Identification |
|---|---|---|---|---|---|---|
| 1 | α-pinene | 3.79 | 940 | 939 | 0.1 | RI, EI-MS |
| 2 | camphene | 4.06 | 950 | 953 | tc | RI, EI-MS |
| 3 | sabinene | 4.52 | 973 | 975 | t | RI, EI-MS |
| 4 | β-pinene | 4.61 | 978 | 979 | 1.1 | RI, EI-MS |
| 5 | myrcene | 4.86 | 990 | 991 | 0.1 | RI, EI-MS |
| 6 | limonene | 5.74 | 1029 | 1029 | 0.3 | RI, EI-MS |
| 7 | γ-terpinene | 6.48 | 1059 | 1062 | t | RI, EI-MS |
| 8 | m-cresol | 6.95 | 1076 | 1077 | 0.4 | RI, EI-MS |
| 9 | terpinolene | 7.37 | 1087 | 1088 | 0.1 | RI, EI-MS |
| 10 | nonanal | 7.72 | 1102 | 1102 | 0.1 | RI, EI-MS |
| 11 | ethyldimethylthiophene | 9.69 | 1170 | - | 1.2 | EI-MS |
| 12 | methyl chavicol | 10.51 | 1195 | 1195 | 0.2 | RI, EI-MS |
| 13 | chavicol | 12.3 | 1254 | 1253 | 1.4 | RI, EI-MS |
| 14 | perilla aldehyde | 12.64 | 1268 | 1272 | 3.5 | RI, EI-MS |
| 15 | bornyl acetate | 13.25 | 1283 | 1285 | 0.4 | RI, EI-MS |
| 16 | α-ylangene | 16 | 1371 | 1372 | 0.3 | RI, EI-MS |
| 17 | β-elemene | 16.53 | 1387 | 1391 | 0.4 | RI, EI-MS |
| 18 | cyperene | 16.72 | 1394 | 1398 | 0.5 | RI, EI-MS |
| 19 | methyl eugenol | 16.97 | 1401 | 1401 | 0.1 | RI, EI-MS |
| 20 | β-caryophyllene | 17.35 | 1414 | 1418 | 2.9 | RI, EI-MS |
| 21 | aromadendrene | 18.23 | 1444 | 1441 | 0.4 | RI, EI-MS |
| 22 | α-humulene | 18.35 | 1449 | 1454 | 0.2 | RI, EI-MS |
| 23 | trans-β-farnesene | 18.56 | 1455 | 1458 | 0.8 | RI, EI-MS |
| 24 | drima-7,9(11)-diene | 19.11 | 1473 | 1473 | 1.5 | RI, EI-MS |
| 25 | germacrene D | 19.27 | 1482 | 1485 | 7.6 | RI, EI-MS |
| 26 | β-selinene | 19.53 | 1486 | 1490 | 1.6 | RI, EI-MS |
| 27 | bicyclogermacrene | 19.75 | 1495 | 1496 | 8.9 | RI, EI-MS |
| 28 | α-selinene | 19.94 | 1499 | 1498 | 0.7 | RI, EI-MS |
| 29 | cis-α-bisabolene | 20.05 | 1503 | 1504 | t | RI, EI-MS |
| 30 | γ-cadinene | 20.27 | 1511 | 1514 | 0.2 | RI, EI-MS |
| 31 | cis-γ- bisabolene | 20.39 | 1516 | 1515 | 0.3 | RI, EI-MS |
| 32 | trans-γ- bisabolene | 21.05 | 1539 | 1531 | 0.5 | RI, EI-MS |
| 33 | spathulenol | 22.02 | 1572 | 1576 | 0.9 | RI, EI-MS |
| 34 | new compound (cmpd. 1)e | 22.88 | 1601 | - | 56.7 | NMR, HREI-MS |
| 35 | foeniculin | 24.8 | 1673 | 1678 | 0.3 | RI, EI-MS |
| 36 | cis-γ-atlantone | 25.11 | 1689 | 1694 | 1.2 | RI, EI-MS |
RI= Calculated retention indices on HP-5 GC capillary column;
RI= Reference retention indices;
Percentages calculated from TIC data;
t = trace (<0.05%);
4-(prop-2-enyl)-phenyl-3'-methylbutyrate.
The unknown compound was assigned the molecular formula C14H18O2 based on HREI-MS positive mode m/z 218.1296 (calc. for C14H18O2, 218.1307, Δ -4.8 ppm), in agreement with the number of carbons and hydrogens in the NMR spectra (Table 1). The IR absorptions indicated the peaks of carbonyl (1759 cm-1), C-O (1203-1101 cm-1), aromatic or olefinic bonds (3080, 1639, and 1506), with no free hydroxyl group. The 13C NMR spectrum supported the existence of the aromatic ring showing six carbon peaks δC 149.0 (s, C-1), 121.5 (d, C-2, C-6), 129.5 (d, C-3, C-5), and 137.2 (s, C-4) at the aromatic region. The carbon resonances were assigned by the use of HSQC spectrum. The 1H-NMR spectrum showed AA'XX' spin pattern of p-disubstiuted aromatic rings for H-2, 6, and H-3, 5 at δH 6.92 (2 × H, d, J = 8.4 Hz, A, A' of AA'XX'), and 7.11 (2 × H, d, J = 8.4 Hz, X, X' of AA'XX'), respectively. In addition, NMR signals indicated the presence of 3'-methyl butanoyl moiety at δC 171.7, 43.4 (δH 2.35 d, J = 7.2, 2 × H); 25.9 (δH 2.18 m, 1 × H); 22.4 (δH 0.98 d, J = 6.8 Hz, 2 × 3H) and an allyl group at δC 39.6 (δH 3.30 d, J = 6.4, 2 × H, H-1''); 137.2 (δH 5.88 m, 1 × H, H-2''); 116.0 (δH 5.03 dd, J = 18.4, 1.81Hz, H-3''a/ 5.01 dd, J = 10.8, 1.81Hz, H-3''b). 1H-1H COSY, as well as HMBC correlations (Figure 1), confirmed the coupling between the protons and characteristic connectivities of the sidechain C1''-H (δH 3.30) with C-4 (δC 137.2) of the aromatic ring. The structure was also confirmed through EI mass ion fragments at m/z 218 [M], 134, 133 [238-3'MB], 91 [M-3'MB –allyl], 77 [C6H5] (Figure 2), which allowed us to establish the structure as 4-(prop-2-enyl)-phenyl-3'-methylbutyrate. Literature survey revealed that 4-(prop-2-enyl)-phenyl-3'-methylbutyrate is a new compound reported for first time. 4-(Prop-2-enyl)-phenyl angelate, its similar compound differed in the type of ester attached to the phenyl ring, was previously reported from essential oil of fruits of P. isaurica (5).
Taken together, GC Mass analysis and HPLC analysis showed that the oil of the fruits of P. haussknechtii consisted of eight monoterpene hydrocarbons (1.7%), two oxygenated monoterpenes (3.9%), sixteen sesquiterpene hydrocarbons (26.8%), two oxygenated sesquiterpenes (2.1%) and five phenylpropanoids (58.7%). Three other nonterpenic compounds were also consisted 1.7% of the oil. The identified components are listed in order of their elution on the HP-5 GC column (Table 2). 4-(2-Propenyl)-phenyl 3'-methylbutyrate (56.7%), bicyclogermacrene (8.9%), germacrene D (7.6%), perilla aldehyde (3.5%), and β-caryophyllene (2.9%) are the main constituents of the oil.
According to the previous study on essential oil composition of fruits of P. kotschyana grown in Tehran, β-caryophyllene (40.6%), germacren D (11.3%), langipinalol (17.6%) and limonene (7.8%) were the major constituents of the oil (D). The main componenets of fruits of P. kotschyana gathered from central parts of Turkey were also reported as β-caryophyllene (49.3%), α-humulene (11.0%), 12-hydroxy-β-caryophyllene acetate (11.5%) and caryophyllene oxide (3.0%) (B). In contrast, 4-(prop-2-enyl)-phenyl-3'-methylbtyrate (56.7%), and bicyclogermacrene (8.9%) were the main component of the P. haussknechtii fruit oil collected from Lorestan, Iran and β-caryophyllene was present in the minor amounts (2.9%).
Phenylpropanoids found in high contents in the oil of different Pimpinella species, were classified in two groups of propenylphenol-type (4-monosubstituted phenylpropanoid) and pseudoisoeugenol-type (2,5-disubstituted phenylpropanoid) (5) from which 4-(prop-2-enyl)-phenyl-3'-methylbutyrate belongs to first group. Previous studies on the volatile oil of fruits of members of Pimpinella genus showed various compositions. trans-Anethole is the major component (75-95%) of P. anisum which could be affected by the genotype and ecological conditions (22-24). Limonene is reported as the major components of P. affinis (90.5%), P. puberula (82.4%) and P. eriocarpa (49.3%) (26,28). β-Pregeijerene (87.0%), bisabolene (50.8%), β-pinene (25.3%) and methyl eugenol (18.7%) are also reported as the major constituents of the essential oils of P. tragioides, P. aurea, P. tragium and P. barbata, respectively (7, 34-36).
The fruits of P. haussknechtii yielded 1.5% (v/w) of yellowish oil with an aromatic odor. Essential oil yields of fruits of different Pimpinella species are very variable, for example the volatile oil yields of fruits of P. cretica var. arabica and P. isaurica are 10.0% and 0.3%, respectively. There are also other species that their fruits have no volatile oil (5).