Introduction
Artemisia genus (Asteraceae) with common Persian name of "Dermaneh" includes 200-400 species in the world that 34 species of it belong to Iran (1). Plants of this genus have been widely used in Iranian Traditional Medicine (ITM) as carminative, diuretic, anthelmintic and also as a cure for insect bites, difficulty in breathing, cramps and fever (2). Modern pharmacological researches have been indicated antimalarial (3-5), antibacterial (6), antifungal (7-8), antiviral (9-10), antioxidant (11), anti-inflammatory (12-14), antipyretic (14), analgesic (12), antiproliferative (15-16), cytotoxic (17), anticancer (18) activities by various species of Artemisia genus. Various phytochemical constituents such as sesquiterpenoids, flavonoids, coumarins, triterpenoids, steroids, purines, phenolics, lipids and aliphatic compounds and monoterpenoids (19-22) were isolated from different species of this genus. Artemisia spicigera known as ''dermane ye sonbolei'' in Persian language, is an aromatic herb growing in Northern and North-western parts of Iran (1). According to previous studies antioxidant, total phenol and flavonoid contents of this plant have been reported (23). In fact, the results of mentioned article indicated that 40% and 60% methanol in water fractions demonstrated maximum antioxidant, total phenol and flavonoids content. So far, however there has been no report on phenolic compounds of this plant, thus the purpose of this paper is to focus on identification of phenolics from methanolic extract of A. spicigera.
Experimental
Plant material
Arial parts of Artemisia spicigera C. Koch were collected from Julfa and border of Aras River in East Azarbaijan province, Iran in 2009. A Voucher specimen for this collection (TBZ-FPH 716) has been deposited at the Herbarium of faculty of pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran.
Extraction
Air dried and powdered arial parts of A. spicigera (100 g) were successively soxhelet extracted using n-hexan (8 h), dichloromethan (DCM) (10 h) and methanol (MeOH) (8 h). Obtained extracts were separately concentrated under vaccum by rotary evaporator at an ambient temperature yielding 6.41 g, 1.45 g and 8.63 g from each extract respectively.
Fractionation of methanolic extract
A portion of dried methanolic extract (2 g) was fractionated by solid-phase-extraction (SPE) on Sep-Pak (Vac 35 cc; 10 g; C18 cartridge) using a step gradient of MeOH-water mixture (10: 90, 20: 80, 40: 60, 60: 40, 80: 20 and 100: 0), 200 ml each. Solvents of eluted fractions were removed using a rotary evaporator at a temperature not exceeding 45ºC. In order to increase the available SPE fractions, procedure was repeated with extra 2 g of methanolic extract yielding 1570, 444, 794, 422, 65 and 670 mg of each fraction, respectively.
Isolation and identification of compounds
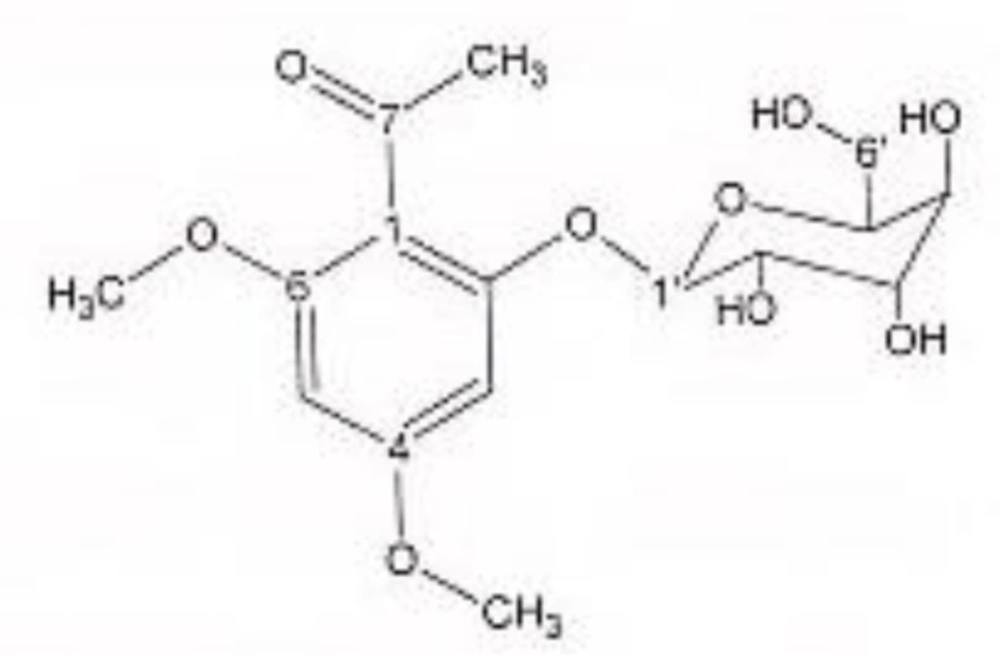
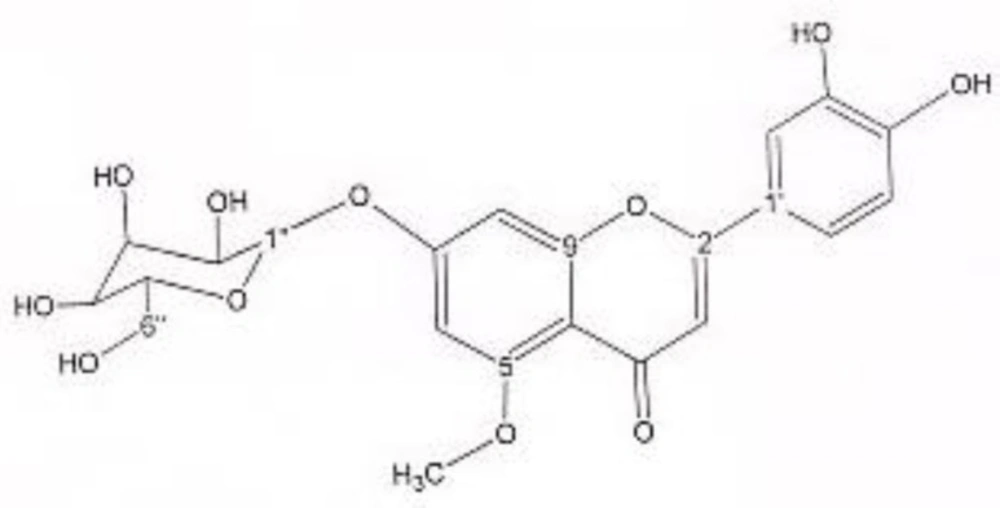
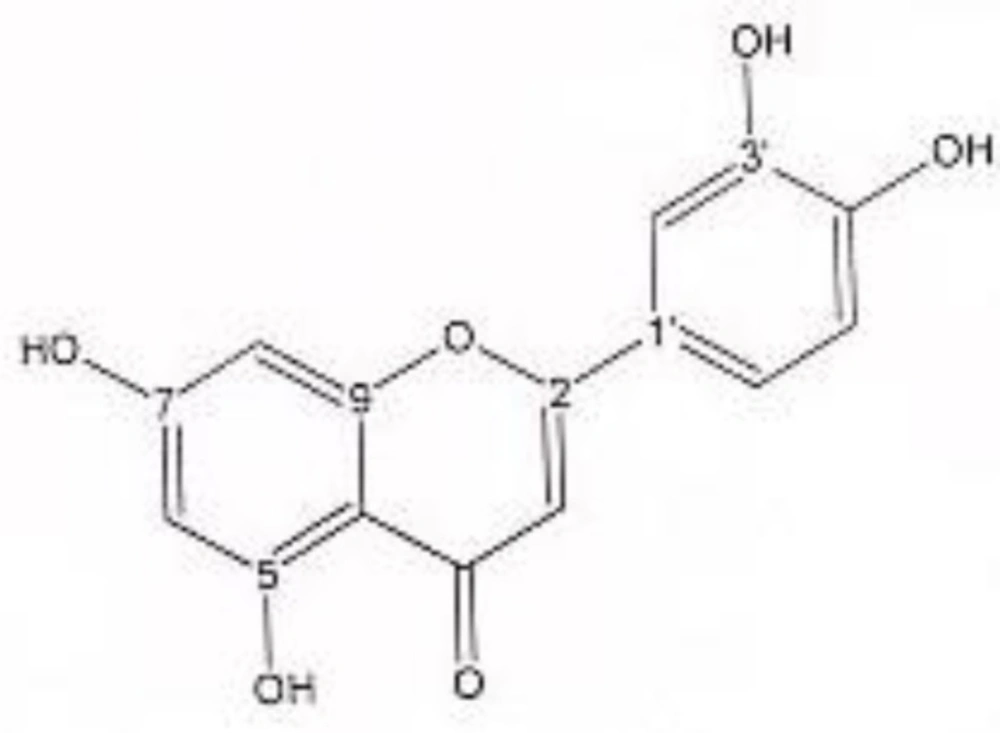
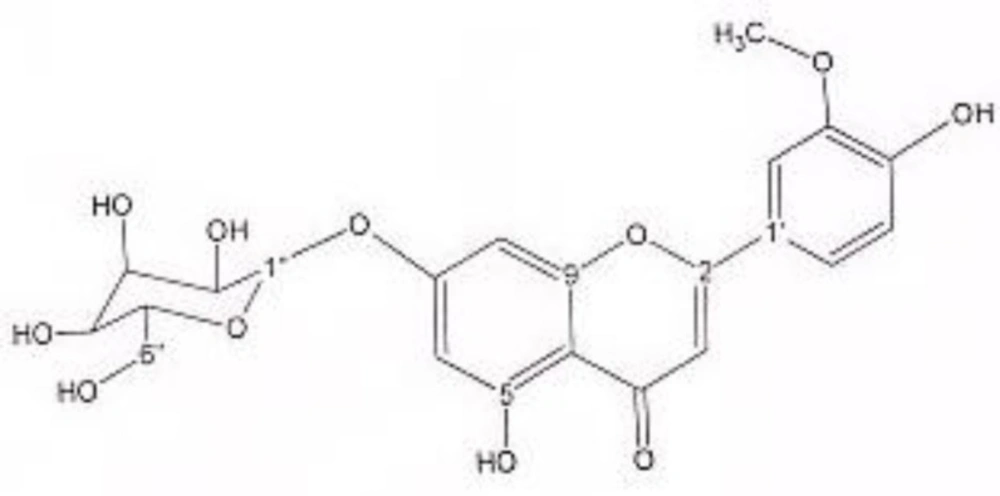
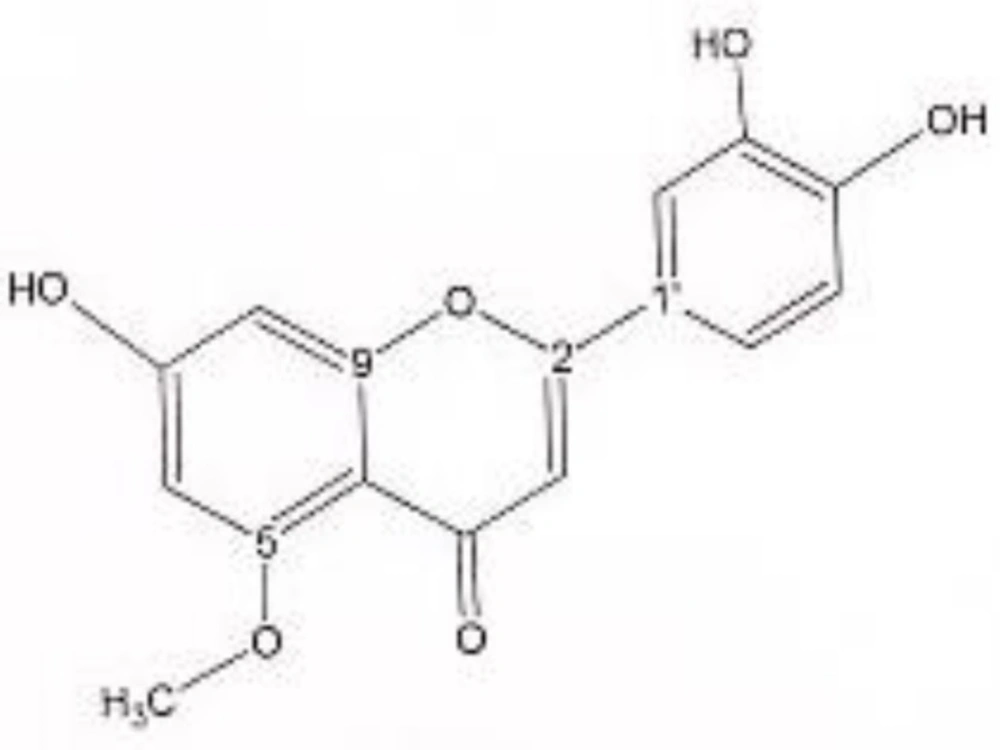
In order to isolate phytochemicals of 20%, 40% and 60% MeOH in water fractions they were subjected to reversed phase preparative HPLC (Knauer, preparative pump 1800),with photodiode array detector (PDA), equipped with a Reprosil 100 C18 (250 mm length, 20 mm i.d, particle size 10 µm, Dr. Maisch, Germany) column. The mobile phase consisted of (A) methanol and (B) water. For isolation of 10.3 mg of 4, 6-di methoxy acetophenone-2-O-β-D- glucopyranoside (Figure 1) from 20% SPE fraction, following mobile phase program was used over 63 min: 15% A was initially changed to 40% within 50 min and maintained there for 13 min. A program over a run time of 63 min was applied for separation of 5.1 mg of 5-methoxyluteolin 7-O-β-D-glucopyranoside (Figure 2), 5 mg of luteolin (Figure 3) and 5 mg of chrysoeriol 7-O-β-D-glucopyranoside (Figure 4) from 40% SPE fraction: 30% A was changed to 80% within 50 min and it stayed there for 13 min. A program over a run time of 63 min was applied for separation of 9.6 mg of 5-methoxy luteolin (Figure 5) from 60% fraction: 45% A was changed to 75% within 30 min and it maintained there for 33 min. The flow rate was 8 ml/min and the injection volume was 1 ml. Structures of compounds were elucidated by 1H and 13C NMR (Bruker-spectrospin at 200 MHz) as well as comparison with the literature data of respective compounds (24-29). Furthermore Ultra Violet spectral analysis of flavonoids by UV spectrophotometer instrument (Shimadzu 1800), as well as shift reagents confirmed structures of identified ones by establishing the site of attachments (30). Shift reagents were as follows: Sodium Methoxide (NaOMe) (Merck), Aluminum Chloride (AlCl3), Aluminum Chloride/Chloridric acid (AlCl3/HCl) (Merck) Sodium Acetate (NaOAC) and Sodium Acetate/Boric acid (NaOAC/H3BO3) (Merck).
1HNMR and 13CNMR of 4, 6-di methoxy acetophenone-2-O-β-D-glucoside was as follows (Figure. 1): HNMR (200 MHz, CD3OD): Aglycone moiety: δ 6.50 (1H, d, J = 2.03 Hz, H3), δ 6.32 (1H, d, J = 2.03 Hz, H5), δ 3.81 (3H, S, 9-OMe), δ 3.79 (3H, S, 10-OMe), δ 2.49 (3H, S, H8). Glucose moiety: δ 4.37 (lH, d, J = 7.8Hz, Hl'), 3.2-3.8 (signal patterns unclear due to over lapping, H2'-3'-4'-5'-6'). 13CNMR (200 MHz, CD3OD). Aglycone moiety: δ 198.01 (C7), 162.07 (C2), 161.25 (C6), 159.33 (C4), 113.04 (C1), δ 94.03 (C5), δ 92.67 (C3), δ 54.94 (C9-OMe), δ 54.66 (C10-OMe), δ 31.50 (C8). Glucose moiety: δ 102.50 (C1'), δ 77.08 (C3'), δ 76.59 (C5'), δ 73.48 (C2'), δ 69.95 (C4'), δ 62.5 (C6').
1HNMR and 13CNMR of 5-methoxyluteolin 7-O-β-D-glucopyranoside was as follows (Figure 2): HNMR (200 MHz, CD3OD): Aglycone moiety: δ 7.58 (1H, dd, J = 8.20, 2 Hz, H6'), δ 7.52 (1H, d, J = 2 Hz, H2'), δ 6.96 (1H, d, J = 8.20 Hz, H5'), δ6.85 (1H, d, J = 2 Hz, H8), δ 6.73 (1H, S, H3), δ 6.50 (1H, d, J = 2 Hz, H6), δ 3.97 (3H, S, O-Me). Glucose moiety: δ 4.39 (lH, d, J = 7.8Hz, Hl''), 3.2-3.9 (signal patterns unclear due to over lapping, H2''-3''-4''-5''-6''). 13CNMR (200 MHz, CD3OD). Aglycone moiety: δ 182.39 (C4), δ 164.85 (C7), δ 164.62 (C2), δ 161.77 (C5), δ 157.93 (C9), δ 149.55 (C4'), δ 145.59 (C3'), δ 122.20 (C1'), δ 118.88 (C6'), δ 115.37 (C5'), δ 112.74 (C2'), δ 102.93 (C10), δ 102.43(C3), δ 98.71 (C6), δ 93.59 (C8), δ 56.96 (C5-OMe). Glucose moiety: δ 98.15 (C1''), δ 76.98 (C5''), δ 76.54 (C3''), δ 72.45 (C2''), δ 70.93 (C4''), δ 62.99 (C6''). UV spectrum, bands ІІ and І respectively (MeOH, λmax, nm): 273, 330; + NaOMe 262, 382; + AlCl3 267, 355; + AlCl3/HCl 273, 330; + NaOAC 271, 375; + NaOAC/H3BO3 265, 355. UV spectrum of compound 2 with MeOH as solvent is characteristic of flavone derivatives. Studying UV spectra data after addition of NaOMe and production of 52 nm band І bathochromic shift, is indicative of 4'-OH. Addition of AlCl3 produced 25 nm band І bathochromic shift. After addition of HCl band І returned to previous model. Thus ortho di-hydroxyl structure of compound can be elucidated. Addition of NaOAC did not produce any band ІІ bathochromic shift which was consistant with glycosylation of 7-OH.
1HNMR and 13CNMR of luteolin was as follows (Figure 3): Aglycone moiety: δ 7.58 (1H, dd, J = 7.95, 2 Hz, H6'), δ 7.50 (1H, d, J = 2 Hz, H2'), δ 6.96 (1H, d, J = 7.95 Hz, H5'), δ6.46 (1H, d, J = 2 Hz, H8), δ 6.63 (1H, S, H3), δ 6.21 (1H, d, J = 2 Hz, H6). Aglycone moiety: δ 182.49 (C4), δ 164.85 (C7), δ 164.63 (C2), δ 161.70 (C5), δ 157.93 (C9), δ 149.55 (C4'), δ 145.59 (C3'), δ 122.20 (C1'), δ 118.85 (C6'), δ 115.32 (C5'), δ 112.69 (C2'), δ 103.00 (C10), δ 102.37 (C3), δ 97.81 (C6), δ 93.50 (C8). UV spectrum, bands ІІ and І respectively (MeOH, λmax, nm): 268, 340; + NaOMe 267, 402; + AlCl3 274, 418; + AlCl3/ HCl 278, 385; + NaOAC 273, 396; + NaOAC/H3BO3 267, 360. UV spectrum of compound 2 with MeOH as solvent is characteristic of flavone derivatives. Studying UV spectra data after addition of NaOMe and production of 62 nm band І bathochromic shift was consistant with 4'-OH. Addition of AlCl3 produced 78 nm band І bathochromic shift. Addition of HCl, caused band І to return to previous model, indicative of ortho di-hydroxyl structure of compound. Addition of NaOAC and production of band ІІ bathochromic shift of 5 nm indicated free 7-OH position of flavonoid (no glycosylation).
1HNMR and 13CNMR of chrysoeriol 7-O-beta-D-glucoside was as follows (Figure 4): HNMR (200 MHz, CD3OD): Aglycone moiety: δ 7.52 (1H, dd, J = 7.95Hz, 2 Hz, H6'), δ 7.50 (1H, S, H2'), δ 6.96 (1H, d, J = 7.95 Hz, H5'), δ 6.73 (1H, S, H3), δ 6.46 (1H, d, J = 2 Hz, H8), δ 6.21 (1H, d, J = 2 Hz, H6). Glucose moiety: δ 4.41 (lH, d, J =7.8Hz, Hl''), 3.2-3.9 (signal patterns unclear due to over lapping, H2''-3''-4''-5''-6''). 13CNMR (200 MHz, CD3OD). Aglycone moiety: δ 181.65 (C4), δ 164.42 (C2), δ 160.7 (C5), δ 163.59 (C7), δ 159.93 (C9), δ 148.55 (C4'), δ 146.52 (C3'), δ 123.26 (C1'), δ 117.88 (C6'), δ 115.67 (C5'), δ 111.74 (C2'), δ 103.43 (C10), δ 102.53(C3), δ 96.90 (C6), δ 95.59 (C8), δ 56.01 (O-Me). Glucose moiety: δ 98.15 (C1''), δ 77.00 (C5''), δ 76.44 (C3''), δ 72.65 (C2''), δ 70.86 (C4''), δ 63.01 (C6''). UV spectrum bands ІІ and І respectively (MeOH, λmax, nm): 277, 340; + NaOMe 282, 400; + AlCl3 274, 355; + AlCl3/HCl 273, 353; + NaOAC 277; + NaOAC/H3BO3 277, 340. UV spectrum of compound 2 with MeOH as solvent is characteristic of flavones derivatives. Studying UV spectra data after addition of NaOMe and production of 60 nm band І bathochromic shift, is indicative of 4'-OH. After addition of AlCl3 and production of 15 nm band І bathochromic shift and no change in absorption maxima after addition of HCl, exhibited that this compound did not consist of ortho di-hydroxyl structure. Addition of NaOAC did not produce any band ІІ bathochromic shift indicating glycosylation of 7-OH position.
1HNMR and 13CNMR of 5-methoxy luteolin was as follows (Figure 5): Aglycone moiety: δ 7.39 (1H, dd, J = 8.30, 2 Hz, H6'), δ 7.37 (1H, d, J = 2 Hz, H2'), δ 6.96 (1H, d, J = 8.30 Hz, H5'), δ6.51 (1H, S, H3), δ6.39 (1H, d, J = 2 Hz, H8), δ 6.16 (1H, d, J = 2 Hz, H6), δ 3.89 (O-Me). Aglycone moiety: δ 182.49 (C4), δ 164.33 (C2), δ 163.85 (C7), δ 159.93 (C9), δ 159.70 (C5), δ 147.45 (C4'), δ 144.50 (C3'), δ 123.50 (C1'), δ 117.85 (C6'), δ 116.32 (C5'), δ 113.68 (C2'), δ 105.00 (C10), δ 103.37 (C3), δ 95.81 (C6), δ 94.50 (C8), δ 56.23 (O-Me). UV spectrum: bands ІІ and І respectively (MeOH, λmax, nm): 268, 330; + NaOMe 276, 387; + AlCl3 274, 414; + AlCl3/HCl 279, 333; + NaOAC 273, 400; + NaOAC/H3BO3 267. UV spectrum of compound 2 with MeOH as solvent is characteristic of flavones derivatives. Studying UV spectra data after addition of NaOMe and production of band І bathochromic shift of 57 nm, is consistant with 4'-OH. Addition of AlCl3 caused 84 nm bathochromic shift. After addition of HCl, band І returned to previous model, indicative of ortho di-hydroxyl structure of compound. Addition of NaOAC and production of band ІІ bathochromic shift of 5 nm was consistent with 7-OH.
Results and Discussion
Solid phase extraction (SPE) followed by reversed-phase prep-HPLC analysis of the arial parts of the SPE fractions (20%, 40% and 60%) yielded one acetophenone as well as four flavonoids, which were 5-methoxyluteolin 7-O-β-D-glucopyranoside, luteolin, chrysoeriol 7-O-β-D-glucoside and 5-methoxy luteolin. Structural identification of isolated phytochemicals was carried out by UV and NMR analysis and all spectroscopic data were in agreement with respective published data. According to our knowledge, none of these phenolic compounds were isolated from A. spicigera. Although in prior studies sesquiterpene lactones have been reported from this plant (31), this is the first report on the isolation and identification of phenolic compounds from A. spicigera. In previous studies, antioxidant activity by DPPH assay, as well as total phenol and total flavonoid content of the MeOH extract of A. spicigera and its solid phase extracted fractions, has been reported. Results of this research showed that proportion of flavonoids and total phenols of 40% and 60% SPE fractions was higher, compared to other fractions and the mentioned active fractions indicated better antioxidant activity as well. Since good radical scavenging activity is associated with different classes of plant phenolics (32-36) so findings of this study is parallel with results of previous works. It means that isolation and identification of phenolics which are one acetophenone and four flavonoids from 20%, 40% and 60% SPE fractions justified higher antioxidant activity as well as higher total phenol and flavonoid content of these fractions.
Acetophenone derivatives are highly distributed in different species of Artemisia genus (37-42), but to our knowledge, 4, 6-dimethoxyacetophenone-2-O-β--glucopyranoside was isolated and identified from Artemisia genus for the first time. The second compound, 5-methoxyluteolin 7-O-β-D-glucopyranoside, was previously isolated from Urtica dioica Maxim. (25), while this was the first report on isolation of this flavonoid from Artemisia genus. Within flavonoids Not only 5-methoxyluteolin 7-O-β-D-glucopyranoside but also chrysoeriol 7-glucoside has not been reported from this genus. Instead, within the genus Artemisia distribution of flavonoids, especially luteolin appears to be widespread (42-45).
Conclusion
It can be concluded that pharmacodynamics studies should be undertaken to establish the mechanism of action of isolated compounds for understanding the reason of their usage in traditional and folk medicine. Hence, further phytochemical investigations will be proposed in order to isolate some other active fractions and pure compounds.