Introduction
Diabetes mellitus is a worldwide epidemic with considerable health and economic consequences that affects nearly 10% of the population (1, 2). This serious metabolic disorder remains a leading cause of cardiovascular diseases, blindness, end-stage renal failure, and hospitalizations. The disease characterized by chronic hyperglycemia and disturbances in fat and protein metabolism (3). Hyperglycemia is typically accompanied by progressive development of microvascular and macrovascular complications, causing morbidity and mortality in diabetic patients (4).
Diabetes is also associated with abnormalities in serum lipids and it was shown that elevated levels of total cholesterol, triglyceride, low density lipoprotein cholesterol (LDL-C) as well as low concentration of high density lipoprotein cholesterol (HDL-C) in diabetes are accompanied with coronary heart disease (5, 6). Therefore, lipid disorders should be quickly diagnosed and treated as a part of diabetes comprehensive treatment. Previous studies have also revealed that the liver is one of the main organs affected by diabetes and that this progressive disease may increase the risk of both chronic liver diseases and hepatocellular carcinoma (7-11). As a consequence, the activities of liver damage markers including, serum alanine aminotransferase (ALT), aspartate transaminase (AST), and alkaline phosphatase (ALP) are increased in the untreated diabetic patients (12). Diabetic nephropathy and renal dysfunction is one the diabetes related complications and approximately 20-40% of patients with diabetes (type 1 and type 2) develop nephropathy (13). As a result, the plasma levels of urea and creatinine are elevated in the diabetic hyperglycemia that is considered as significant markers of renal impairment (14).
Medicinal plants have become increasingly attractive agents in the treatment of diabetes and its related complications (15-17). Cydonia oblonga Mill.,commonly called quince is one of these plants that belongs to the Rosaceae family and is cultivated for fruit production all over the world. It is believed that quince originated in Northern Iran, Turkmenistan, and the far west regions of Anatolia and Greece (18). Cydonia oblonga Mill. Fruit is recognized as an important dietary source and contains polyphenolic compounds, organic acids, and free amino acids (19-21). Cydonia oblonga Mill. has been utilized to decrease symptoms of diabetes in Iranian and Turkish folk medicines and it was shown that the leaves of this plant has antioxidant, anti-hyperglycemic, hypolipidaemic, and hepatoprotective effects (22-23). Since hyperglycemia is accompanied by dyslipidemia and abnormalities in liver and kidney functions, the present study was designed to investigate the hypolipidemic, hepatoprotective, and renoprotective properties of Cydonia oblonga Mill. Fruit aqueous extract in streptozotocin-induced diabetic rats.
Experimental
Materials and Methods
Plant material and preparation of the extract: Cydonia oblonga Mill. Fruits were collected from Shahriar, Alborz province of Iran. The collected fruits were scientifically approved by the Department of Botany, Shahid Beheshti University (Voucher number: 8054, deposited in: Shahid Beheshti University Herbarium). Fresh fruits were cleaned and then dried in the shade at room temperature. Fruits were decocted in water for 30 min. Then, the extract was filtered and concentrated to the desired level (honey-like viscosity), and stored at -20°C. The moisture level of the extract was determined as follows: 2 g of final extract was placed in an oven at 60–65°C for 72 h and then weighed. Weight loss was used as a moisture indicator. The final extract contained 24% water. This extract was dissolved in distilled water at the desired concentrations just before use (24). We chose a wide concentration range for aqueous extract of Cydonia oblonga Mill. Fruit in our pilot study and their inhibitory effects were evaluated (data not shown). By omitting non effective, poor effective or toxic concentrations, the 80, 160 and 240 mg/kg body weight were selected.
Chemicals: Streptozotocin was purchased from Sigma-Aldrich Co. (Taufkrichen, Germany). All other chemicals were of the highest commercial grade available.
Animals: Male Sprague-Dawley rats weighing 250 to 300 g were housed in ventilated plastic cages over PWI 8-16 hardwood bedding. There were 12 air changes per h, 12 h light photoperiod (lights on at 0800 h), an environmental temperature of 21–23°C and a relative humidity of 50–60%. The animals were fed a normal standard chow diet and given tap water ad libitum. Principles of laboratory animal care" (NIH publication No. 85-23, revised 1985) were followed. All experiments were conducted according to the ethical standards and protocols approved by the Committee of Animal Experimentation of Zanjan University of Medical Sciences, Zanjan, Iran.
Experimental induction of diabetes in rats: Experimental diabetes was induced following an overnight fast, by a single intraperitoneal (i.p.) injection of 60 mg/kg streptozotocin freshly dissolved in 1.0 ml citrate buffer (0.1 M, pH = 4.5) (25). Control animals were injected with vehicle only. Hyperglycemia was confirmed 4 days after injection by measuring the tail vein blood glucose level with a standardized glucometer (ARKAY, INC., Japan). Only the animals with fasting blood glucose levels ≥ 250 mg/dl were selected for the study.
Collection of blood samples: Blood samples were collected from the tail vein. Blood was collected and serum was immediately frozen and stored at -70°C until the biochemical determinations were performed.
Biochemical parameters: The serum levels of triglyceride, total cholesterol, HDL-C, ALT, AST, ALP, urea, and creatinine were determined by commercially available enzyme kits (Pars Azmoon, Tehran, Iran) and using an automatic analyzer (Architect c8000 Clinical Chemistry System, USA) (26). LDL-C was calculated according to Friedewald equation as follows: LDL-C = Total cholesterol-HDL-C- (Triglyceride/5) (27).
Protocol design: Normal and diabetic rats were divided into five groups of nine rats each (n = 9). Group I served as negative control (non diabetic); Group II served as positive control (diabetic but not treated); Group III, IV and V diabetic rats were treated with aqueous extract of Cydonia oblonga Mill. Fruit at 80, 160, and 240 mg/kg body weight respectively. Each plant extract sample was administered orally once daily using an intragastric tube for 6 weeks.
Statistical Analysis: Levene’s test was used to check the homogeneity of variances. The data were analysed using one-way analysis of variance (ANOVA) followed by Tukey’s HSD as the post hoc test. The results were presented as the mean ± SD (n = 9). The minimal level of significance chosen was p < 0.05.
Results and Discussion
Serum lipid profiles
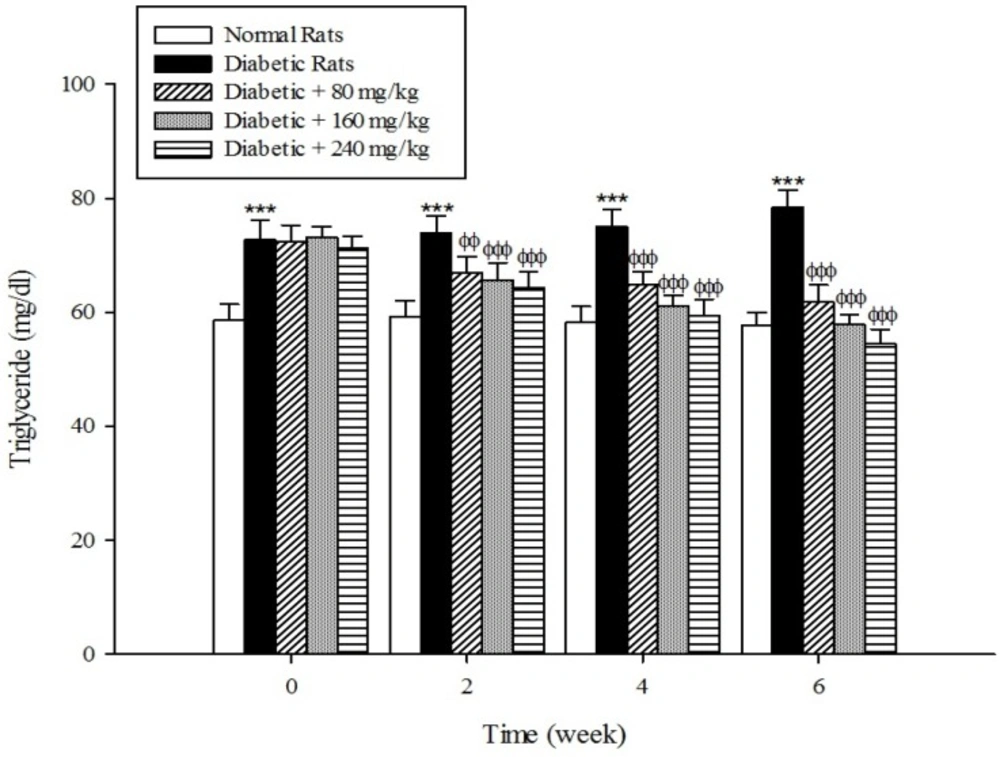
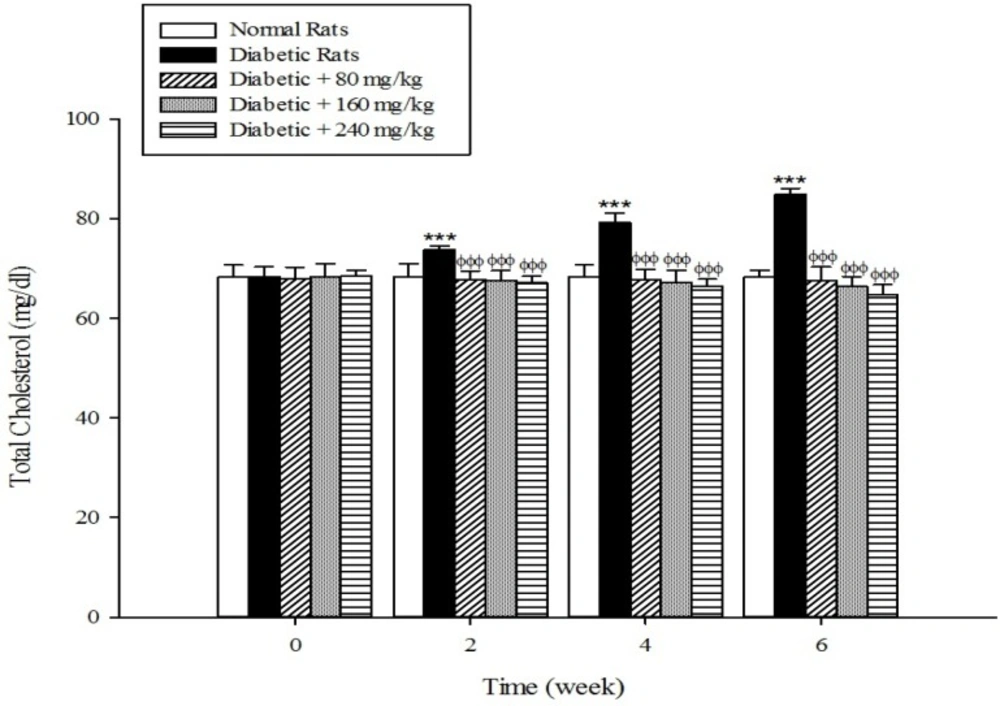
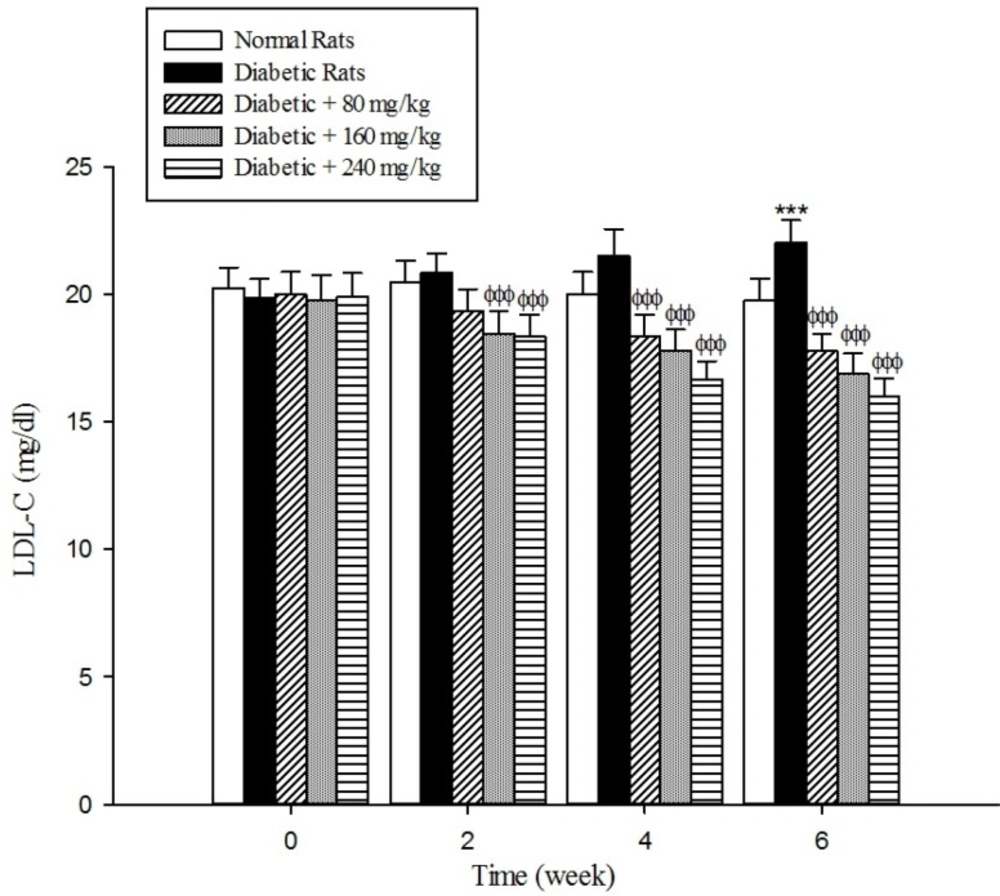
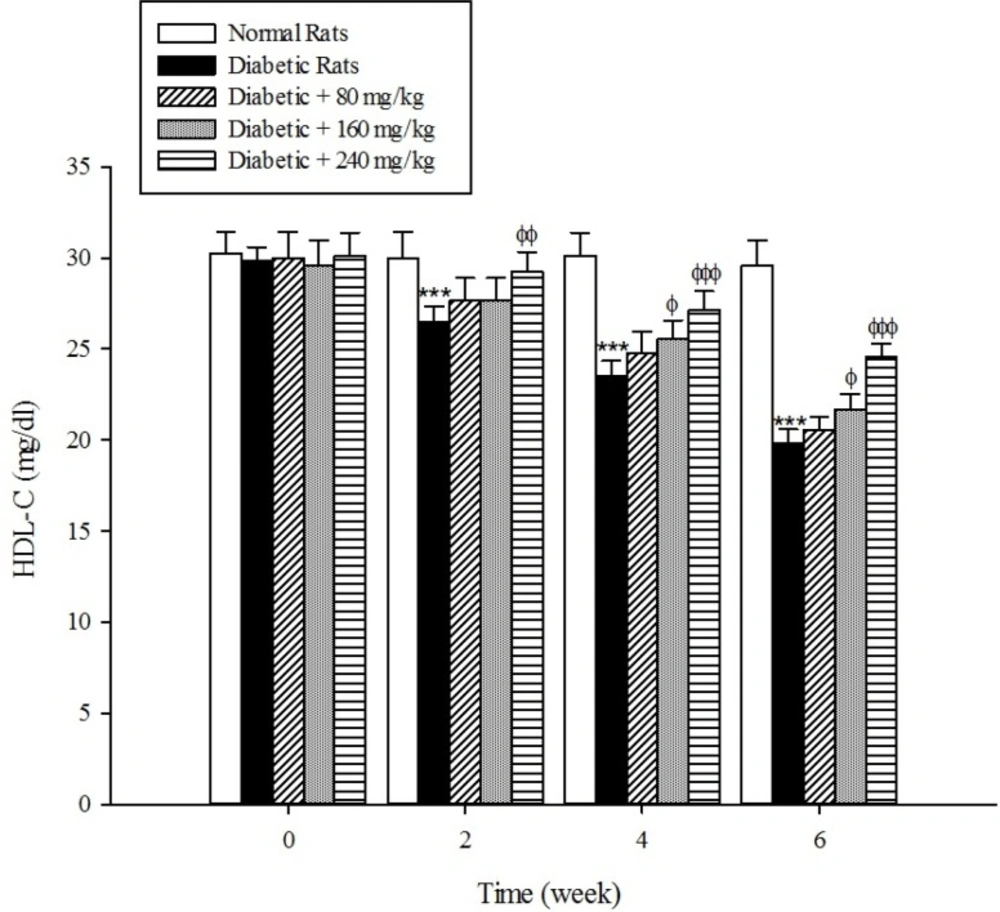
The results of the serum lipid profile showed that streptozotocin injection led to the development of hyperlipidemia in which serum triglyceride, total cholesterol, and LDL-C markedly (p < 0.001) increased when compared to the control group (Figure 1-3). However, HDL-C decreased in diabetic rats in comparison with the normal control (Figure 4). As shown in Figure1-4 the different concentrations of aqueous extract of Cydonia oblonga Mill. Fruit caused a significant decrease in the serum triglyceride, total cholesterol, and LDL-C, but a significant increase in HDL-C levels in the diabetic rats during 6 weeks of the treatment.
Liver parameters
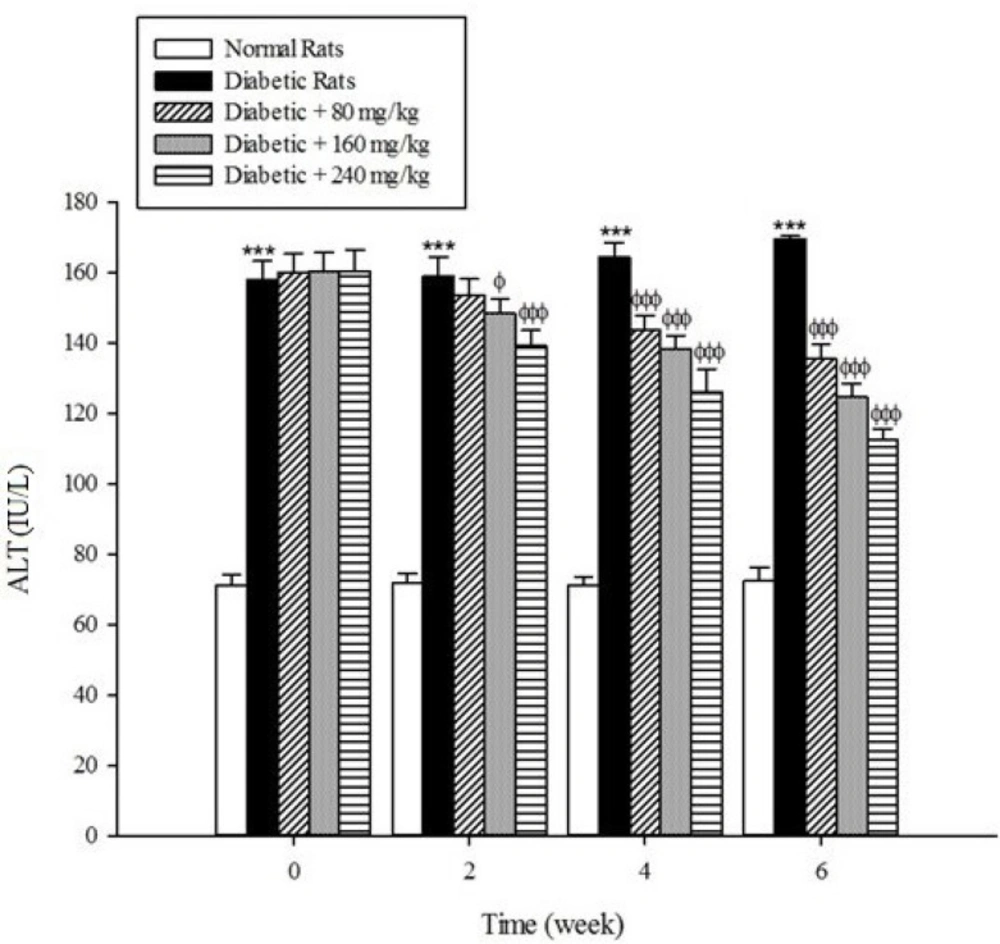
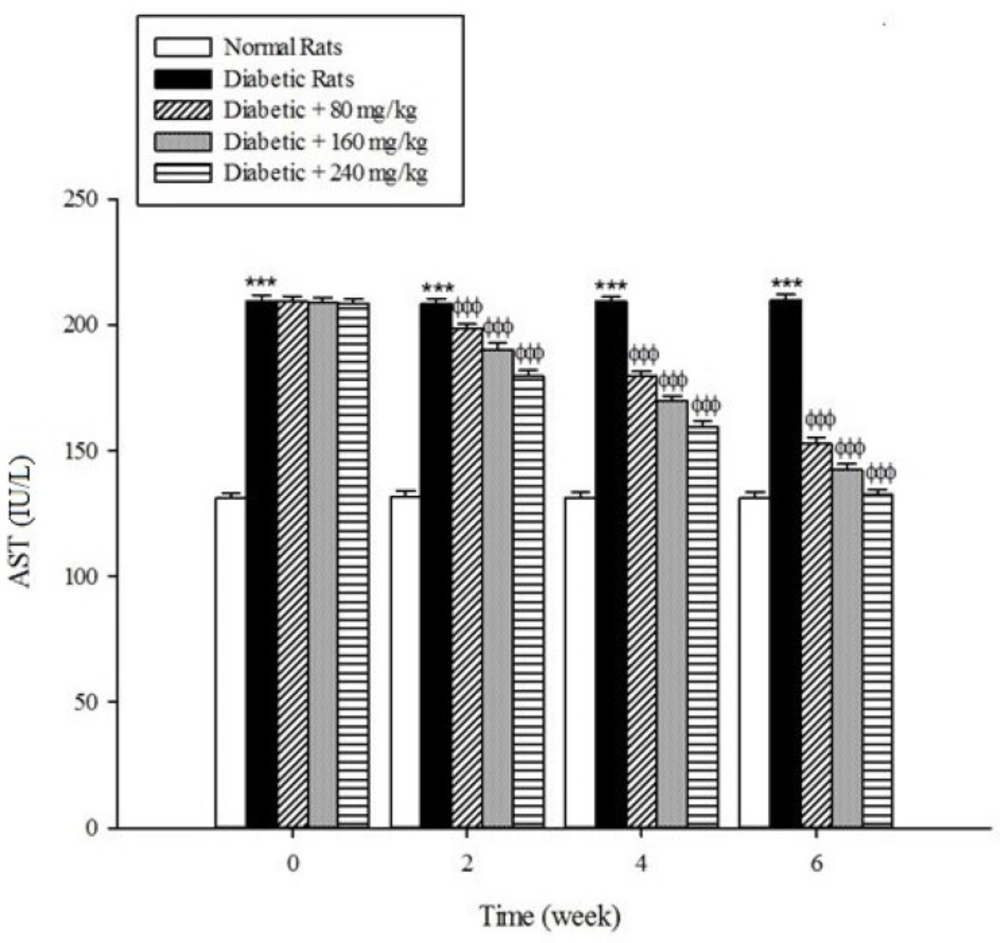
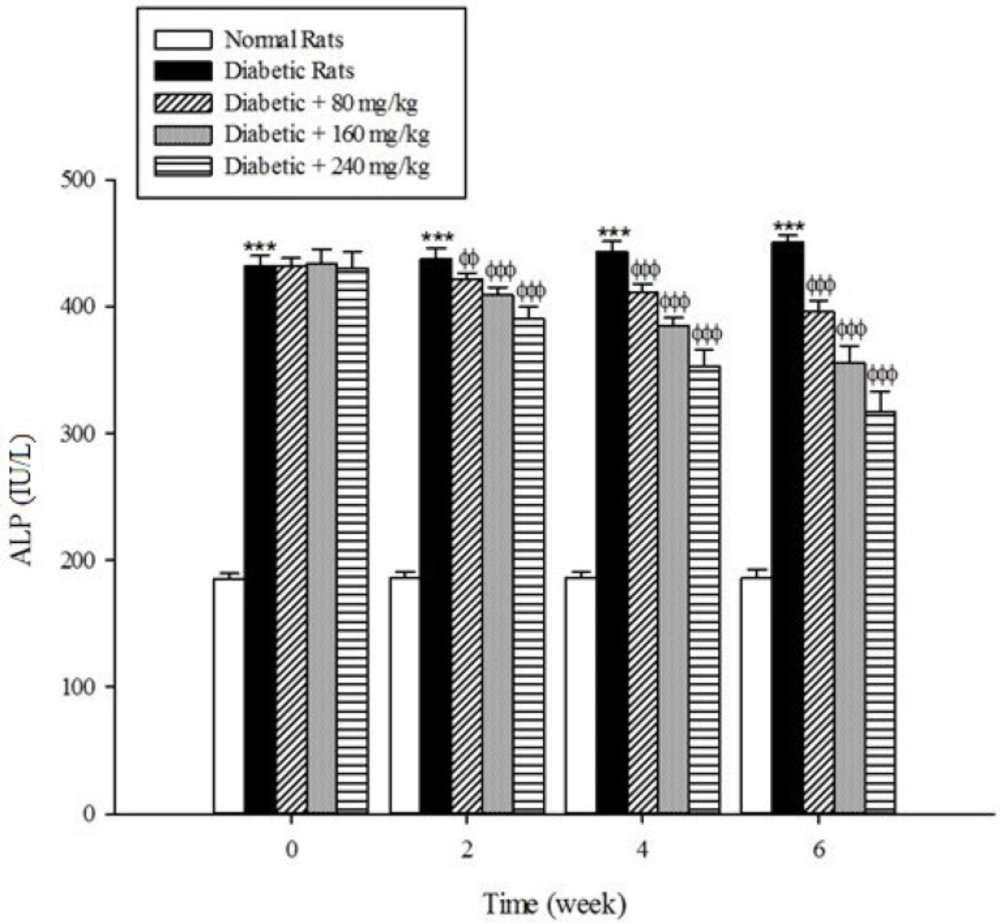
Serum activities of ALT, AST, and ALP as the markers of liver function significantly (p < 0.001) were increased in the untreated diabetic rats in comparison to the non-diabetic rats (Figure 5-7). The extract at the concentrations of 80, 160, and 240mg/kg caused a significant decrease in the biomarkers of liver injury in the diabetic rats treated with the extract (p < 0.001).
Kidney parameters
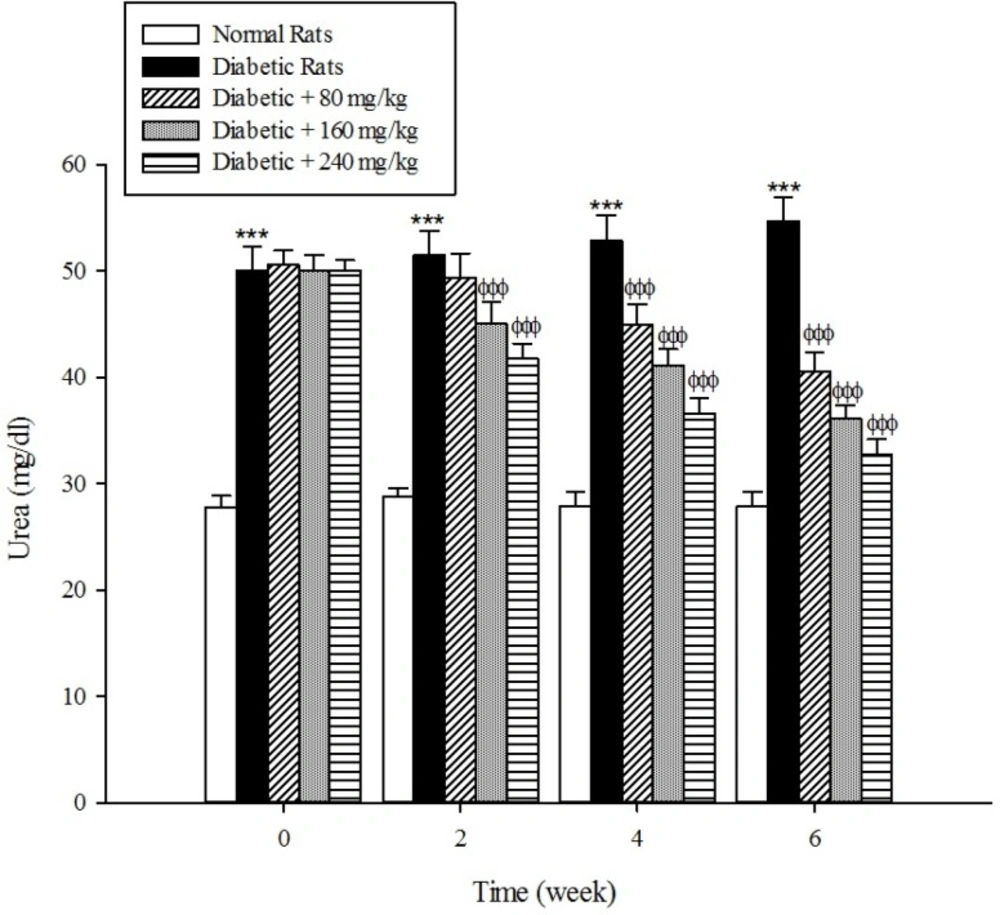
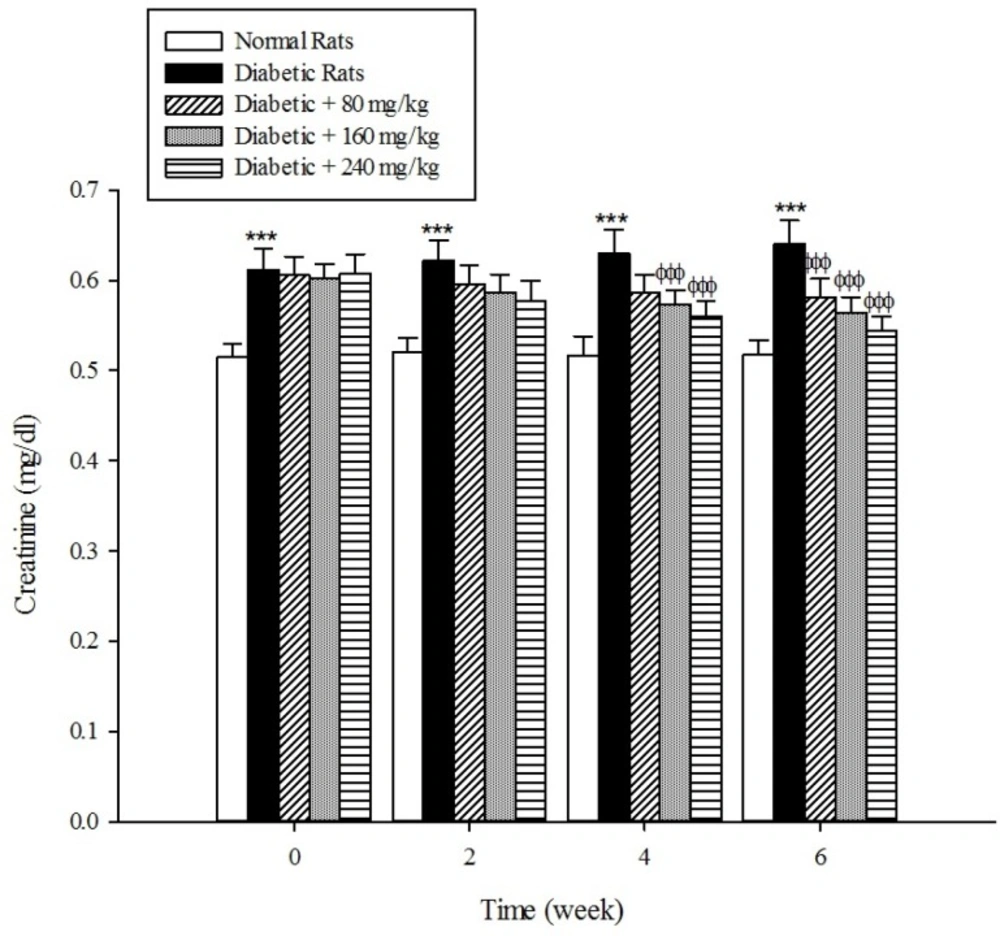
The levels of serum blood urea and creatinine as the markers of renal dysfunction were elevated (p < 0.001) in the diabetic rats when compared with the normal rats (Figure 8, 9). As shown in Fig 8 and 9 the treatment of diabetic animals with the extract significantly inhibited an increase in the serum urea and creatinine concentrations (p < 0.001).
Drug-induced diabetes is one of the most commonly used experimental diabetic models (28). In the present study diabetes was induced in rats by injection of streptozotocin. Diabetes is often accompanied by hyperlipidemia that manifests marked elevations of cholesterol, triglyceride, and LDL-C as well as low concentration of HDL-C (29, 30). These serum lipid abnormalities result due to disruption of fatty acid metabolism (31). Our results confirm that hyperlipidemia was occurred in the diabetic rats. Natural products that reduce or alter serum lipid profiles have proved to be effective for the treatment of many diabetic complications (32). Our findings showed that the oral administration of aqueous extract of Cydonia oblonga Mill. Fruit was able to ameliorate serum lipid profiles in the diabetic rats. It can be therefore suggested that quince fruit could be a potential source of hypolipidemic agent (s) and it can be used in the management of hyperlipidemia in diabetic patients.
Diabetes plays a central role in the initiation and progression of liver injury and this progressive disease is an independent risk factor for the development of chronic liver diseases (33, 34). The serum activities of ALT, AST, and ALP are biomarkers of hepatic injury (15, 35). ALT and AST are transaminase enzymes that catalyse amino transfer reactions and play an important role in amino acids catabolism and biosynthesis (36, 37). In addition, ALP is a hydrolase enzyme which acts as non-specific phosphomonoesterases to hydrolyse phosphate esters (38). In the present study, the serum elevation of liver damage biomarkers was occurred as a result of deleterious effect of hyperglycemia in the liver of diabetic rats. Increasing the activities of these enzymes is mainly due to leakage of the enzymes from the liver into the blood stream as a result of streptozotocin toxicity which leads to the liver damage. However, the treatment of diabetic groups with the extract of Cydonia oblonga Mill. for 42 consecutive days could ameliorate the activities of the above enzymes. A possible explanation for the hepatoprotective effects of the extract is that this fruit may inhibit the liver damage induced by streptozotocin. These results suggest a hepatoprotective role for quince fruit against liver injury associated with diabetes.
Diabetes mellitus is also associated with complications in the renal system. Patients with diabetes experience major long-term complications such as nephropathy and diabetic nephropathy is one of the leading causes of end-stage renal disease (ESRD) in the world (39, 40). Our results reconfirmed that the plasma levels of urea and creatinine, which are considered as significant biomarkers of renal dysfunction (41), were increased in the experimentally induced-diabetes. However, the treatment of diabetic rats with the extract of Cydonia oblonga Mill. reduced their plasma urea and creatinine levels. This implies that quince fruit normalizes the function of kidneys in the diabetic rats.
It was reported that the extract of Cydonia oblonga Mill. leaves possessed remarkable hypoglycemic effect in streptozotocin-induced diabetic rats. The leaves extract also showed antioxidant activity and protected the heart tissue against lipid peroxides produced by diabetes (23). In addition, Cydonia oblonga Mill. Leaf extracts showed hypolipidaemic and hepatoprotective effects in the rat model of hyperlipidaemia (22). Our results demonstrated that the fruit of Cydonia oblonga Mill. possesses hypolipidemic, hepatoprotective, and renoprotective effects in streptozotocin-induced diabetic rats. Previous studies have also shown that Cydonia oblonga Mill. Fruit contains polyphenols (19). It is well established that polyphenolic compounds have hypoglycemic activity and prevent the development of diabetic complications (42, 43). Therefore, the presence of these constituents may explain the protective effects of this fruit in diabetes-related complications. However, we believe that further studies are necessary to determine the exact nature of the active components and the mechanism of action of Cydonia oblonga Mill. Fruit in diabetes and its associated complications.
Conclusion
The results of this study demonstrate that the oral administration of aqueous extract of Cydonia oblonga Mill. Fruit improve serum lipid profile in diabetic rats by lowering cholesterol, triglyceride, and LDL-C levels and raising HDL-C levels. In addition, the hepatoprotective effect of quince fruit is demonstrated by the significant reduction of serum levels of ALT, AST, and ALP in the diabetic treated rats. The extract also improved renal function in diabetic rats by reducing serum urea and creatinine. It can be concluded that Cydonia oblonga Mill. Fruit possesses hypolipidemic, hepatoprotective, and renoprotective effects in streptozotocin-induced diabetic rats.