Introduction
Free radicals have been claimed to play an important role in affecting human health by causing many diseases (e.g., heart diseases, cancer, hypertension, diabetes and atherosclerosis). In the past decade, anti-oxidants have shown their relevance in the prevention of various diseases, in which free radicals are implicated (1).
According to the previous studies, terrestrial plants are rich sources of phytochemicals possessing important properties such as antioxidant activity. Many investigators have found several types of anti-oxidants from different parts of various plant species such as oilseeds, cereal crops, vegetables and spices (2).
Recently, polyphenolic compounds including flavonoids is known as safe and non-toxic anti-oxidants. Many studies have shown that a high dietary intake of natural phenolics is strongly associated with longer life expectancy, reduced risk of developing some chronic diseases, various types of cancer, diabetes, obesity, improved endothelial function and reduced blood pressure (3-5). Phenolic compounds are commonly found in plants and seaweeds. Like other plants, seaweeds contain various inorganic and organic substances, which can benefit human health (6). It has been observed that ROS production in algae is stimulated by various environmental stresses, such as high light levels, heavy metals, high salt concentrations, UV radiation etc. Algae generally has higher antioxidant activity due to a higher contents of nonenzymatic antioxidant components, such as ascorbic acid, reduced glutathione, phenols and flavonoids (7). As a result, many marine bio-sources in the last decades have attracted attention in the search for natural bioactive compounds to develop new drugs and healthy foods. Compounds with antioxidant, antiviral, antifungal, antimicrobial, antitumor and anti-inflammatory activities have been found in brown, red and green algae (8).
The antioxidant activity of several seaweeds has been reported (9, 10). Ulva genus, an edible seaweed, and an important food source in many south-east Asian countries is also recognized by its synonymous name as Enteromorpha. To the best of our knowledge, there is no publication on the antioxidant activities of green seaweeds from Iran. The present study aimed to investigate the antioxidant properties of four Ulva species from the northern coasts of the Persian Gulf for future applications in medicine, dietary supplements, cosmetics or food industries.
Experimental
Chemicals
Ascorbic acid, Folin-ciocalteu reagent, Gallic acid and Methanol were purchased from Merck Company (Darmstadt, Germany). DPPH and Rutin were purchased from Sigma Chemical Co (St.Louis, MO, USA). All the chemicals and reagents used were of analytical grade.
Collection and preparing of algal extract
The seaweeds were collected at low tide time(according to the tide time table obtained from www.iranhydrography.org) along the northern coasts of the Persian Gulf, from Dayyer, Taheri and Northern Ouli (Figure 1) in April 2011. The latitude and longitude of each sampling location was recorded by GPS tracking device.
Once harvested, seaweeds were washed with fresh water to remove sands, salts and epiphytes, and then, were air-dried at room temperature with good controlled air condition carefully. The algae samples were pressed and stored in 5% formol for identification. Voucher specimens were deposited in Jundishapur Marine Pharmaceutical Research Center herbarium. Morphological and anatomical examinations of cell structures were done with the aid of stereomicroscope and light microscope. The samples were identified according to the characteristics and identification keys in the taxonomic publications (11-15). Samples kept at -50 ºC until experiments were processed and milled into powder before extraction.
Dried seaweed sample powder (200 mg) was extracted with 6 mL 80% methanol in an ultrasonic bath for 20 min, vortexed for 30 min and then left to stand at room temperature for 48 h. The extract centrifuged at 1500 g for 10 min, filtered through Watmann No.1 filter paper and then, was freeze dried. The dried extracts were weighed and the yield of each extract was calculated. The stock solutions of the extracts were adjusted with 80% methanol to final concentration of 2 mg (dry extract) mL-1. Dilutions were made to obtain concentrations 1, 0.5 and 0.1 mg mL-1.
DPPH free radical scavenging activity
DPPH radical scavenging activity was determined according to the method of Zhang et al. (2007) with slight modifications (16). Briefly, 100 µL of each extract at various dilutions, were mixed with 100 µL of 0.16 mM DPPH solution .The mixture was vortexed for 1 min, kept for 30 min in dark and then, the absorbance was measured at 517 nm in an automated microplate reader (Sunrise-Elisa Reader, Tecan, Swiss). The antioxidant capacity was calculated using the following equation:
% Inhibition = (Acotrol - (Asample - Ablank) / Acotrol) × 100
Where the Acotrol is the absorbance of the control (DPPH without sample), the Asample is the absorbance of the test sample (the sample test and DPPH solution), and the Ablank is the absorbance of the sample blank (Sample without the DPPH solution). The half-maximal inhibitory concentration (IC50) was calculated by linear regression analysis and expressed as mean of three determinations. Ascorbic acid was used as positive control.
Determination of total phenolic compounds and flavonoid content
Total phenolic compounds (TPC) of algal extracts was determined by Folin-Ciocalteu reagent according to the method of Antolovich et al. (2002) (17) with minor modifications. In Brief, 20 µL of extracts were mixed with 100 µL of 1:10 Folin-Ciocalteu reagent followed by the addition of Na2CO3 (80 µL, 7.5%). The assay was carried out in microplate. After incubation at room temperature for 2 hours in dark, the absorbance at 600 nm was recorded. Gallic acid was used as the standard reference. TPC was expressed as mg Gallic acid equivalents per gram of dried extract (mg GAE g-1).
Flavonoid content of each extract was determined by following colorimetric method (18). Briefly, 20 µL of each extract were separately mixed with 20 µL of 10 % aluminium chloride, 20 µL of 1 M potassium acetate and 180 µL of distilled water, and left at room temperature for 30 min. The absorbance of the reaction was recorded at 415 nm. The calibration curve was prepared by using Rutin methanolic solutions at concentrations of 12.5 to 100 µg mL-1. FC was expressed as mg Rutin equivalents per gram of dried extract (mg RE g-1).
Statistics
Data were expressed as means ± standard errors of three replicate determinations. All statistics analyses were carried out using SPSS 16.0 for Windows. To determine whether there were any differences among the means, one way analysis (ANOVA) and the Duncan’s new multiple range test were applied to the result. p-values < 0.05 were regarded to be significant. The Pearson correlation analysis was performed between antioxidant activity and total phenolic and flavonoids, and also between total phenolic and flavonoid contents.
Results and Discussion
DPPH radical scavenging activity
During the study, four edible Ulva species were collected from northern coasts of the Persian Gulf. U.intestinalis collected from two different locations (Dayyer and Northern Ouli). The species, use and medicinal effects of them and their collection information are listed in Tables 1 and 2. The Extraction yields of samples (S1-S5) were 10.60, 28.43, 20.42, 13.39 and 25.82 %, respectively. Due to the presence of different bioactive components with antioxidative potential in the crude extracts of the samples, many different methods have been used to investigate various samples in recent years. In the current study, the DPPH radical scavenging method used to evaluate the antioxidant capacity of the seaweed extracts, because of reliability of the test (19). All seaweed extracts showed antioxidant activity to various degrees (Table 3). Lower IC50 value indicates higher antioxidant activity. As shown in Table 3, in comparison to the IC50 of ascorbic acid (0.043 ± 0.001 mg ML-1) as a standard antioxidant, U.clathrata (S1) exhibited a relatively high antioxidant activity with a relatively low IC50 ( 0.881 ± 0.047 mg mL-1) which was significantly different (p < 0.05 ) compared with those of the other species.
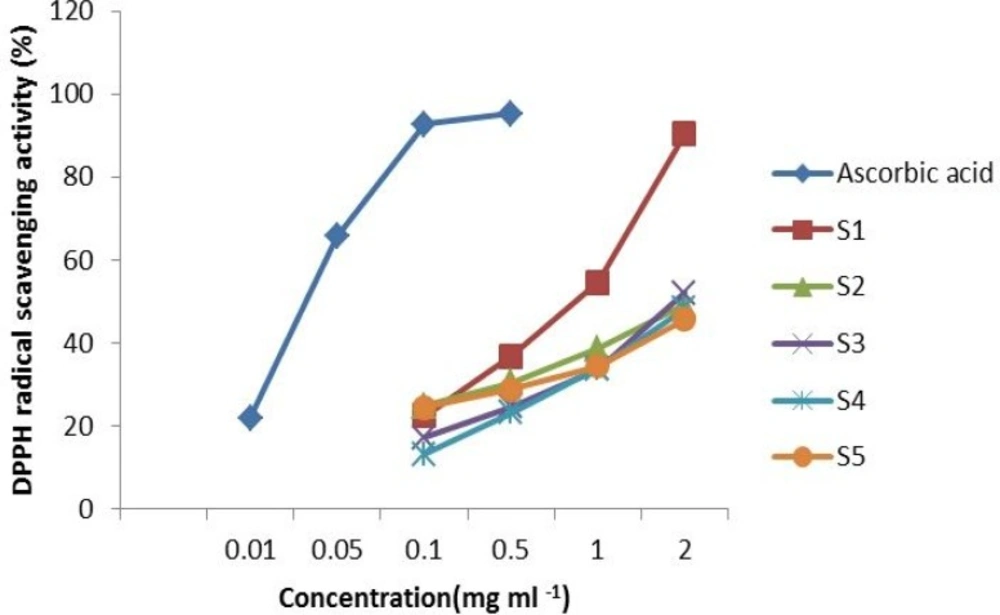
The scavenging effect of the tested extracts at concentration of 2 mg mL-1 on the DPPH radical decreased in the order of: S1 > S2 > S3 > S4 > S5, and were 90.3, 49.19, 52.15, 48.28 and 45.79% , respectively(Figure 2). The inhibitory effect of all extracts were dose dependent in the range of the tested concentrations. As shown in Figure 2, the inhibitory effect increased with increasing concentration. However, the extract of U.clathrata was found to be the most potent scavenger in these tested algae. The activity of the U.clathrata extract (2 mg mL-1) was comparable to that of the positive control, ascorbic acid (at concentration of 0.1 mg mL-1) (p < 0.05).
| Scientific name | Uses/ medicinal effects |
|---|---|
| Ulva clathrata(Roth) C.Agardh | Anti-tumorigenic, blood anticoagulant activity(35, 36) |
| Ulva linza Linnaeus | Antibacterial and anti-inflammatory activity(37, 38) |
| Ulva flexuosa Wulfen | Cytotoxicity against breast ductal carcinoma cell line, high antibacterial activity(39) |
| Ulva intestinalis Linnaeus | Antibacterial and antihemolytic activities (40) |
| Algae | Sample number | Herbarium ID Code | Locality | Latitude, Longitude |
|---|---|---|---|---|
| Ulva clathrata | S1 | G110721 | Taheri | 27º40’04”N- 52º19’71,1”E |
| U.intestinalis | S2 | G110421 | Dayyer | 27º50’01,6”N- 51º56’19,3”E |
| U.linza | S3 | G110921 | Northern Ouli | 27º50’31,6” N- 51º53’08”E |
| U.intestinalis | S4 | G110922 | Northern Ouli | 27º50’31,6” N- 51º53’08”E |
| U.flexuosa | S5 | G110923 | Northern Ouli | 27º50’31,6” N- 51º53’08”E |
Many studies have been done to determine antioxidant capacity in Ulva species. For instance, 48 marine algae were tested for their antioxidant activity and a low antioxidant activity with a relatively high IC50 (43.23 ± 0.28 mg mL-1) were reported for Ulva intestinalis among the all tested seaweeds (20). However, some researchers have stated high scavenging activity for Ulva species.
For example, three edible species of Ulva including U.compressa, U. linza and U. tubulosa exhibited high antioxidant activity in linoleic acid system and the best DPPH radical scavenging was observed in methanolic extract of U. compressa (IC50 = 1.89 mg mL-1) (21). Also, a high value of astaxanthin (a naturally occurring carotenoid pigment and a powerful antioxidant) has been reported in Ulva intestinalis (22). It has been shown that, chronic consumption of polysaccharides supplied by Ulva species, prevent the fall of antioxidant defences and the development of atherosclerosis in hamsters (23). Besides, some researchers have demonstrated that the natural Ulvan (a group of sulfated heteropolysaccharides obtained from Ulva species) and its derivatives exhibited much higher scavenging activity on superoxide radical than vitamin C (24). Moreover, sesquiterpenoids have been isolated from Ulva fasciata with free radical scavenging properties (25). Furthermore, Polysaccharides from U. lactuca extract with antioxidant effects in experimentally-induced hypercholesterolemic animal model have been reported (26).
Total phenolic and flavonoid contents
Total phenolic content (TPC) and flavonoid content (FC) of the algal extracts are also presented in Table 3. The content of phenolic compounds varied from 5.08 ± 0.65 (Ulva clathrata) to 1.258 ± 0.126 (U.intestinalis (S5)) mg GAE g-1.The phenolic content in the U.clathrata extract was significantly different (p < 0.05 ) compared with those of the other species. In general, the higher total phenolic content resulted in higher antioxidant capacity. According to the Table 3, the phenolic content of U.flexuosa and U.intestinalis (S5) which collected from the same location were significantly different(2.674 ± 0.221 and 1.258 ± 0.126, respectively) (p < 0.05 ) and was higher in U.flexuosa. The same result for two Halimeda species (of the same area) is repoted by Yoshie et al. (2001) (27). This difference in polyphenolic contents may be due to local variations.
As shown in Table 3, the flavonoid content of algal extracts varied from 33.094 ± 2.053 (Ulva clathrata) to 8.048 ± 1.119 (U.intestinalis (S5)) mg RE g-1. The flavonoid contents of two samples of U.intestinalis (S3 and S5) were significantly different and were higher in S3 (25.316 ± 2.198 mg RE g-1). Despite the fact that, the same species were from the same collection season, however, contents of their flavonoids were different. Previous studies have found marked changes in the chemical constituents with change of seasons and environmental conditions (28).This variation in flavonoid content may be due to the variation in physicochemical parameters such as salinity amongst the selected stations.
| Algae | Sample | IC50 (mg mL-1) | TPC(mgGAEg-1) | FC (mg RE g-1) |
|---|---|---|---|---|
| Ulva clathrata | S1 | 0.881 ± 0.047 a | 5.080 ± 0.650 a | 33.094 ± 2.053 a |
| U.linza | S2 | 1.819 ± 0.632 b | 1.996 ± 0.298 bc | 10.431 ± 2.215 c |
| U.intestinalis | S3 | 1.881 ± 0.034 b | 1.982 ± 0.308 bc | 25.316 ± 2.198 b |
| U.flexuosa | S4 | 2.175 ± 0.038 b | 2.674 ± 0.221 b | 9.462 ± 1.558 c |
| U.intestinalis | S5 | 2.372 ± 0.022 b | 1.258 ± 0.126 c | 8.048 ± 1.119 c |
The Pearson’s correlation coefficients between the variables are presented in Table 4. As shown in the table, there were strong positive significant correlations between DPPH radical scavenging and contents of phenolics and flavonoids, and high negative correlations between IC50 and the variables. Also, the results revealed that there was a strong positive correlation between flavonoids and total phenolics (r = 0.759, p < 0.01).
| Phenolic content | Flavonoid content | IC50 | |
|---|---|---|---|
| Flavonoid content | 0.759** | - | - |
| IC50 | -0.785** | - 0.804** | - |
| DPPH radical scavenging activity | 0.889** | 0.819** | -0.866** |
The antioxidant activity of Ulva species were in accordance with their amount of total phenolic and flavonoid contents. Several reports have shown a close relationship between total phenolic content and high antioxidant activity, and many researchers have demonstrated that phenolic compounds are one of the most effective antioxidants in marine algae (29, 30).
The best-described property of almost every group of flavonoids is their capacity to act as antioxidants. Flavonoids are oxidized by radicals, resulting in a more stable, less-reactive radical. In other words, flavonoids stabilize the reactive oxygen species by reacting with the reactive compound of the radical (31). A positive correlation has been documented between antioxidation capabilities and total polyphenol contents for Ulva prolifera, but not with the contents of flavonoids (32). In the current study, strong positive correlations were found between total phenol and flavonoid contents and the antioxidant capacity. Similar observation has been reported by Chai and Wong (2012) (33). The current research findings were in agreement with the results of Bouba et al. (2010) which reported a positive correlation between total phenolics and flavonoids in extracts of twenty Cameroonian spices (34). In the current study, only Ulva clathrata was collected from middle intertidal rocks where the seaweeds are exposed to UV radiation for several hours in a day. The other tested seaweeds collected from lower intertidal zones. Prolonged seaweed exposure to solar UV radiation may result in producing bioactive compounds such as phenolics and flavonoids and may be an explanation of higher antioxidant capacity of Ulva clathrata in comparison with the other tested species.
Conclusion
In the current study, the antioxidant activities of four Ulva species were evaluated. The results clearly indicated that all the tested seaweeds in this investigation possess antioxidant activity. Ulva clathrata exhibited high phenolics and flavonoid contents and also, high antioxidant activity with a low IC50. Strong positive and significant correlations between DPPH radical scavenging and phenolics and flavonoid contents showed that, phenolic compounds, including flavonoids are the main contributors of antioxidant activity in these Ulva species. However, to the best of our knowledge, this is the first report of investigation on the antioxidant capacity and total phenolics as well as flavonoid content of Ulva species from Iran. Further work is under way in our laboratories which are aimed at investigation of antioxidant capacity of the other seaweeds of northern coasts of the Persian Gulf and also we are working on the physicochemical parameters of water to find correlation between environment condition and naturally synthesized components by these algae.