Introduction
Olea europaea L. (family Oleaceae), commonly known as olive, is a plant of ancient world and is a typical plant of Mediterranean area with noticeable pharmacological activities (1). O. europaea has been widely used in folk medicine to treat inflammatory diseases such as rheumatoid arthritis and hemorrhoids, and as a vasodilator in vascular disorders (2). The pharmacological properties of olive fruit, leave, and its oil have been recognized as important components of medicine and a healthy diet (3).
O. europaea is rich in bioactive phenolic compounds. Oleuropein is one of the most important phenolic components of the O. europaea, which has broad pharmacological activities including antioxidant, anti-inflammatory, anti-atherogenic, anti-cancer, antimicrobial and antiviral activity, hypolipidemic, and hypoglycemic effects (4).
Oleuropein increases nitric oxide (NO) production under the stress of lipopolysaccharide in macrophages through induction of the enzyme nitric oxide synthase (5). Other studies indicate that olive leaf extract has analgesic property in folk medicine and potentiates the anti-nociceptive effect of morphine (6).
There are reports showing the analgesic and anti-inflammatory activity of olive’s oil (7,8). However, no information is available about the anti-nociceptive and anti-inflammatory effects of the defatted olive fruit extract. In this study, the anti-nociceptive effect of methanolic and aqueous extracts of defatted olive fruit was evaluated by formalin test and the anti-inflammatory activity was tested by measurement of paw edema volume.
Experimental
Plant material
Plant material used in this study was collected in Roudbar, in the northern province of Iran (Gilan). After identifying the samples by a qualified botanist, the voucher specimens were deposited in the Herbarium of Department of Pharmacognosy, Shahid Beheshti University of Medical Sciences, Tehran, Iran (voucher number: 1114).
Preparation of the extracts
Defatted aqueous extract: Olives (1 Kg) were first chopped and extracted with boiling water (1000 mL) then filtered through a two-layer mesh. In order to remove remained oil, water extract was transferred to a decantation apparatus, mixed with petroleum ether and shaken well 3 times, until oil was separated. The water-soluble portion was concentrated almost to dryness and stored in 2-8 ºC until used. The extract was dissolved in normal saline at the desired concentration just before using.
Defatted methanol extract: Olives (1 Kg) were soaked in 1000 mL methanol at room temperature for 48 h. The extract was filtered with filter paper and this process was repeated twice. After that, solvent was removed using rotatory evaporator under vacuum and dry methanol extract was obtained. Then the extract was defatted with petroleum ether.
Extracts were stored in airtight containers at 2-8 ºC for further testing.
Animals
Male Sprague-Dawley rats weighing 150-200 g were obtained from Razi institute, Karaj, Iran. The animals were kept under a 12 h light/dark cycle in a controlled condition at room temperature (22 ± 2 °C) and had free access to food and water. All the procedures were in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and approved by institutional animal care and use committee in Shahid Beheshti University of Medical Sciences. In all experiments tried to use the minimum number of animals.
Formalin test
To determine the anti-nociceptive activity of the extracts, formalin test was used. The procedure was the same as the method described previously (9). Aqueous and methanolic extracts (300, 450, and 600 mg/Kg), sodium salicylate as positive control (300 mg/Kg), and normal saline as control (1 mL/Kg) were given intraperitoneally (i.p), 30 min before running the formalin test. After pretreatments with the extracts, 50 μl of 2.5% formalin in saline was injected subcutaneously into the planar surface of the animal hind paw and the behavior of the animal (favoring, lifting, licking, and shaking of the injured paw) was monitored for 60 min.
To assess the possible participation of the opioid system in the anti-nociceptive effect of the olive fruit extract, naloxone was injected (4 mg/Kg, i.p.) 15 min after injection of the aqueous extract and 15 min before the formalin injection in a separate group of animals.
Paw edema volume
Measurement of rat-hind paw edema was carried out by recording the volume after formalin injection according to the method of Fereidoni et al. (10). After 30 min i.p. administration of the aqueous extract, 50 μL formalin 5% was injected subcutaneous to the hind paw to induce inflammation. The inflamed paw was inserted in a mercury column, which was fixed on a digital balance, to a specific area and the weight was read. The number read represented the weight of displaced mercury. Then, the paw volume is easily calculated using the following formula: V = m/ρ, in which ρ is the fluid density (13.6 g/mL) and m is the changes in weight recorded after insertion of the paw. The difference between the paw volume before and after the inflammation is the edema volume.
Statistical analysis
All data are presented as mean ± SEM. To evaluate the effect of treatment in formalin test the results were analyzed by repeated measures two-way ANOVA and post-test of Bonferroni. The area under the curve of the second phase of formalin test was analyzed by one-way ANOVA and post-hoc analysis of Tukey. Data of paw edema was also analyzed by repeated measures Two-way ANOVA and post-hoc analysis of Bonferroni to evaluate the effect of treatments. In all statistical analysis, P < 0.05 was considered as significant.
Results
Yield of extraction
Calculated HER (herbal to extract ratio) for defatted aqueous extract was approximately 4.84% and for defatted methanol extract was 4.96%.
Formalin test
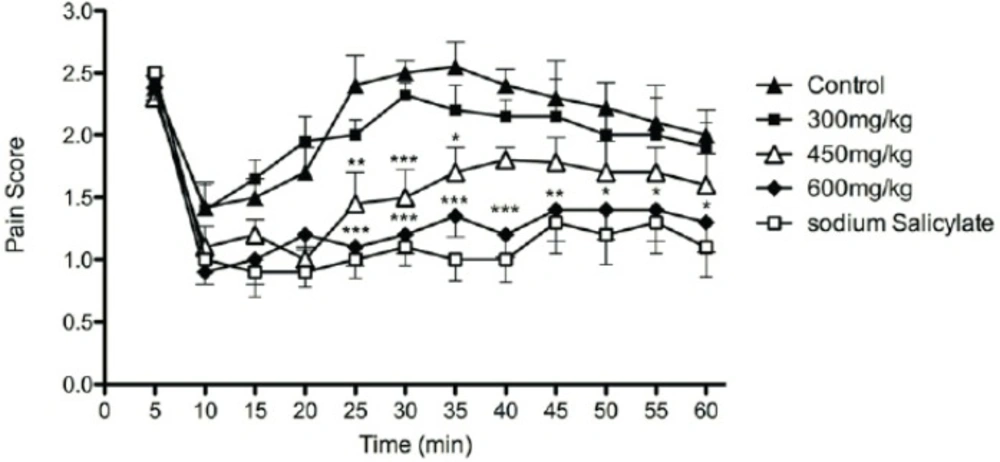
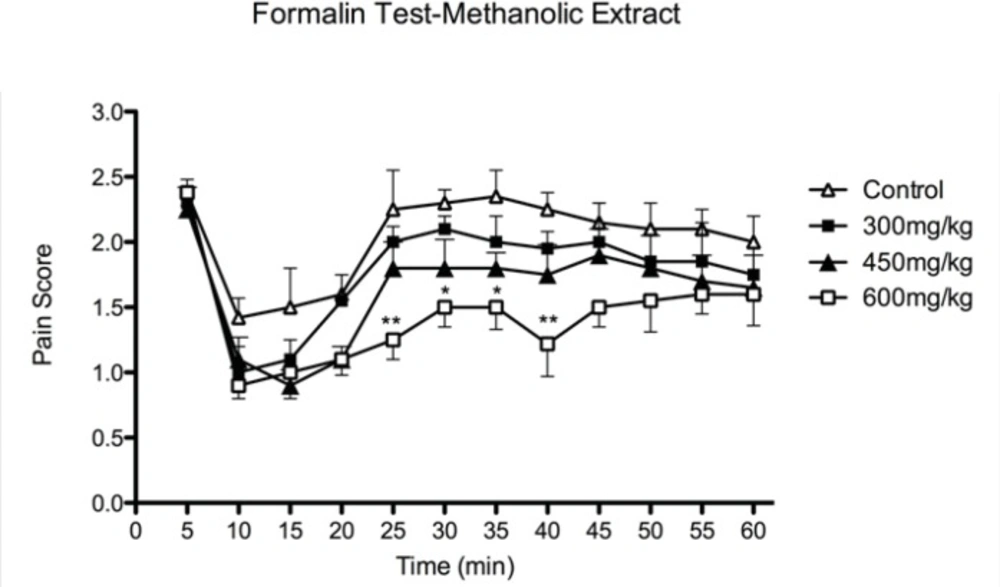
The effect of aqueous and methanolic extracts is shown in Figure 1 and Figure 2 respectively. None of the extracts were effective in the first phase of formalin-induced pain. Results obtained from the effect of extracts at the second phase of formalin test showed a dose dependent anti-nociceptive activity. In this phase of formalin test (15-60 min), methanolic extract at dose of 600 mg/Kg and aqueous extract at doses of 450 and 600 mg/Kg showed anti-nociceptive effects.
The effects of aqueous extract of defatted olive fruit on pain score in formalin test: Aqueous extract at doses of 450 and 600 mg/Kg and sodium salicylate in dose of 300 mg/Kg show anti-nociceptive effects at phase II of the test. The extracts do not show any significant effect at phase I of the test. In all groups n = 7. * represents P < 0.05, ** represents P < 0.01, and *** represents P < 0.001 compared to control
The effects of methanolic extract of defatted olive fruit on pain score in formalin test: Methanolic extract at dose of 600 mg/kg shows anti-nociceptive effects at phase II of the test. The extract does not show any significant effect at phase I of the test. In all groups n = 7. * represents P<0.05 and ** represents P < 0.01 compared to control.
In comparison with sodium salicylate at the dose of 300 mg/Kg, anti-nociceptive activity of aqueous extract of olive fruit at dose of 600 mg/Kg was about the effect of sodium salicylate.
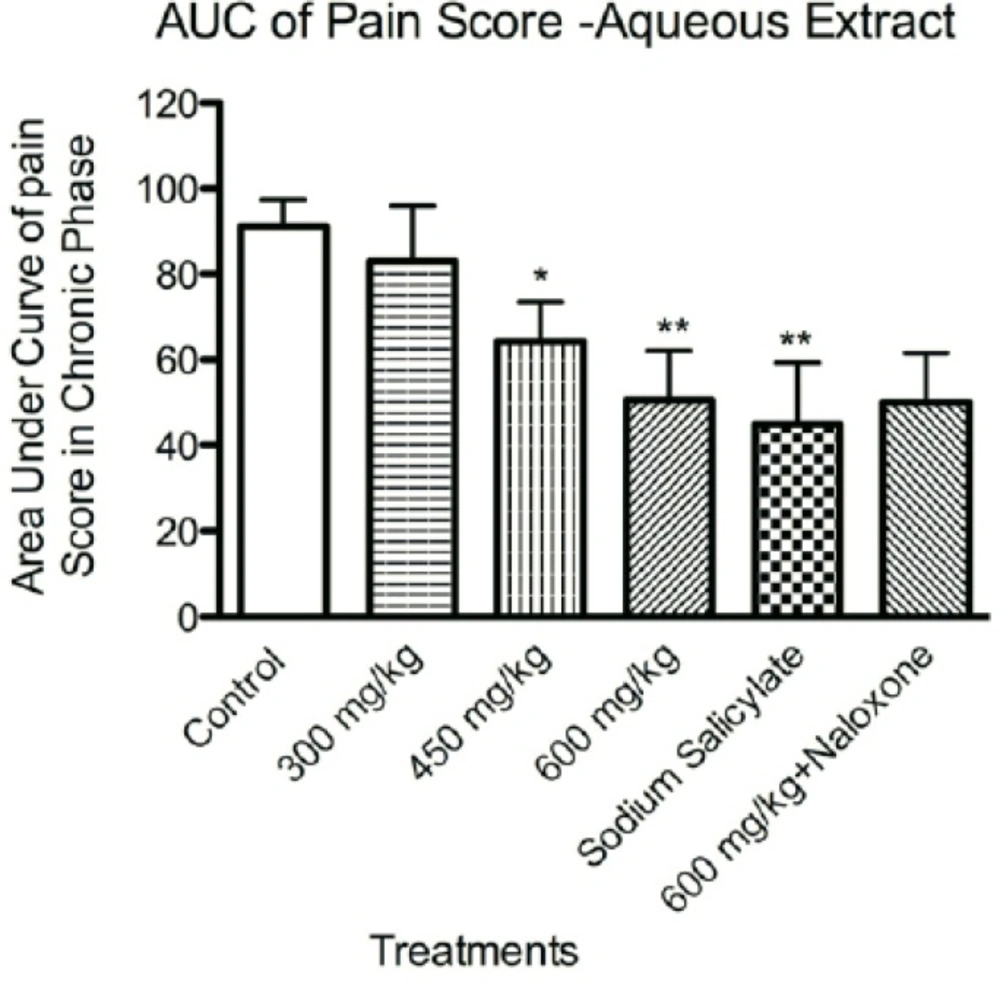
We further investigated whether naloxone could reverse the anti-nociceptive activity of aqueous extract. Data showed that anti-nociceptive effect of aqueous extract in highest effective doses (600 mg/Kg) was not inhibited by pretreatment with naloxone (Figure 3).
The effects of aqueous extract of defatted olive fruit on pain score in formalin test: analyzing the area under the curve of pain score in phase II of the test during the time of experiment shows that the aqueous extract at doses of 450 and 600 mg/Kg and sodium salicylate in dose of 300 mg/Kg have anti-nociceptive effects at phase II of the test. Naloxone was not able to reverse the effect of 600 mg/Kg of the aqueous extract. In all groups n=7. * represents P<0.05 and ** represents P<0.01 compared to control
Paw edema volume
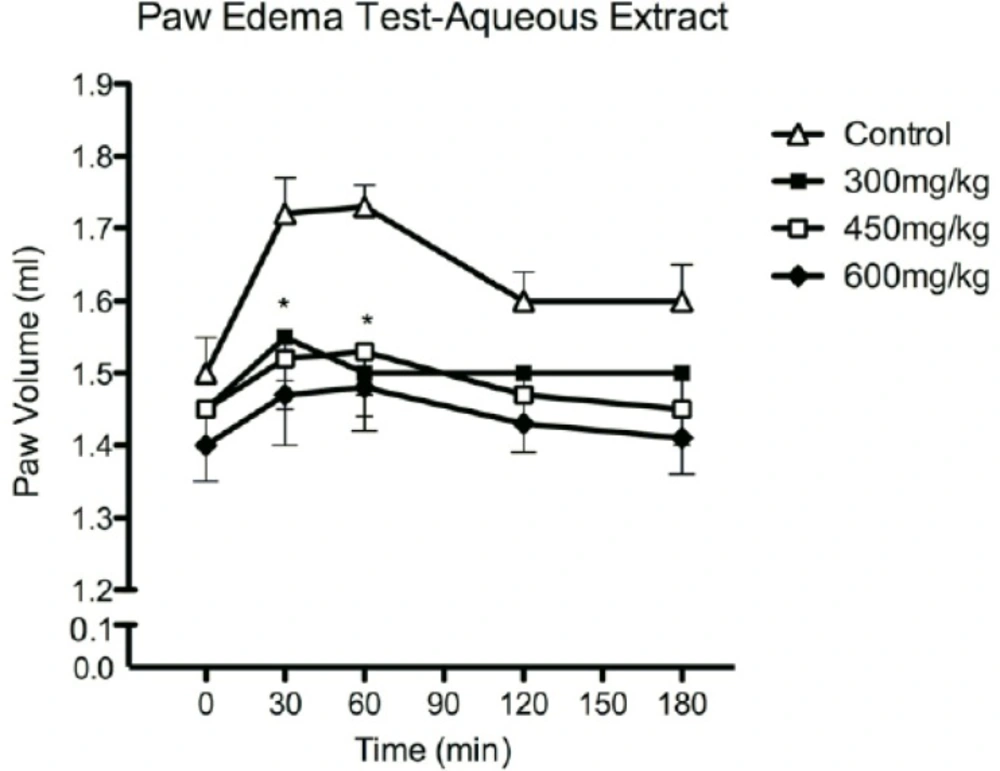
Figure 4 shows the change of rats paw volumes. Results indicated that aqueous extract of olive fruit at the dose of 300, 450 and 600 mg/Kg (P < 0.01) inhibited the formalin induced edema, although differences between 300, 450 and 600 mg/Kg doses of extracts were not significant.
Discussion
Both the aqueous and methanolic extracts of defatted olive fruit showed activity in the second phase of formalin test when compared to sodium salicylate as a standard analgesic drug. The results showed that the aqueous extract was more potent than the methanolic one.
The formalin test is a valid and reliable model of nociception, which is predominantly used on rats and mice. The response to the formalin as a noxious stimulus are favoring, lifting, licking, and shaking of the injured paw by animals. Two distinct periods of high licking activity can be identified, an early phase lasting the first 5 min that seems to be caused predominantly by C-fibre activation due to the peripheral stimulus and a late phase lasting from 15 to 30 min after the injection of formalin, in which different chemical mediators such as PGE2, nitric oxide, kinins, serotonin and histamine are involved (11, 12, 13).
It is accepted that the action of analgesic drugs differs in the two phases of the formalin test. While central acting drugs (opiate analgesics) inhibit both phases, anti-inflammatory drugs (non-opiate analgesics) especially inhibit the second phase (14).
But the anti-nociceptive effect of defatted fruit extract of O. europaea does not involve the opioid system since it was not reversed by pretreatment with naloxone and this analgesic effect could be associated to the anti-inflammatory action and mediated by its peripheral effect.
The aqueous extract of defatted olive fruit also exhibited anti-inflammatory activity in the paw edema induced by formalin test. Thus, the anti-inflammatory action of O. europaea in the formalin paw edema test is in agreement with the results of anti-nociceptive test.
This is the first attempt to evaluate the anti-nociceptive and anti-inflammatory effects of defatted O. europaea fruit, confirming that anti-inflammation and pain-reducing effects of olive are not only related to its oil content. We also suggest that phenolic compounds presented in olive fruits are major contributors to its anti-inflammatory and anti-nociceptive effects. Additional experiments are needed to find the active components(s) and the exact mechanism of action of the component(s).
In conclusion, our results indicate that olive fruit extract inhibits pain and inflammation, which supports the use of this plant in traditional medicine for the treatment of inflammatory diseases.