Introduction
The incidence of cancer in human populations and the increasing need for anti-cancer drugs on the one hand and discovery of effective anti-cancer drugs, such as taxol, vincristine and vinblastin from plants. E.macrostegia as one of the endemic plants to Iran is the subject of this investigation. Euphorbia macrostegia (Persian wood spurge), belongs to the family Euphorbiaceae distributed mostly in central and west parts of Iran. Persian wood spurge is similar to the wood spurge (Euphorbia amygdaloides) and a rare species native of semi-moist woods from south-eastern Europe through Asia Minor. In the Iranian traditional medicine, latex is used to treat warts. Despite their toxicity, the uses of Euphorbia species in traditional medicine in many parts of the world have a long history. They are used to treat inflammations and tumours (1). Previous investigation on the cytotoxicity assessment of E. macrostegia (2), has showed LD50 values of 200, 425, and 390 μgmL− 1 for dichloromethane, ethyl acetate and acetone fractions, respectively while other fractions, remarked as noncytotoxic. Therefore, based on previous studies on cytotoxiciy effects of E. macrostegia and its fractions, the authors decided to investigate phytochemical contents of the dichloromethane extract of this plant as the most active fraction.
Result and Discussion
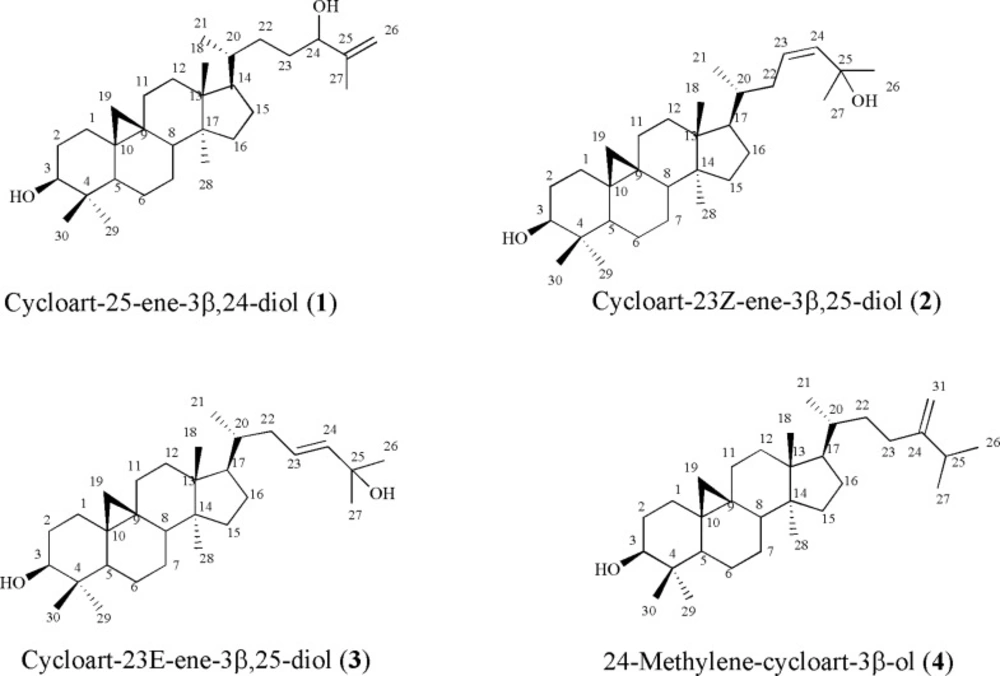
Compound 1, white crystals, showed the molecular formula of C30H50O2 based on EI-MS m/z 442 and number and multiplicity of 13C-NMR spectra. The six-degree of unsaturation and the 13C-NMR data (Table 1), suggested the presence of one double bond and, therefore, a pentacyclic skeleton. EI-MS fragmentation pattern, supported m/z 355 and 302, typical ions of 4,4' dimethyl 9:19 cycloesterols (4). 1H-NMR revealed a pair of doublets in the up-field area 0.57, 0.36 (each 1H, d, J = 4.0 Hz, H-19a, b), characteristic of cycloartane cyclopropane ring (4), one secondary methyl group at 0.90 (3H, d, J = 6.4 Hz, H-21), and five singlet methyls at δH 0.83 (3H, s, H-29), 0.91 (3H, s, H-28), 0.98 (3H, s, H-18), 0.99 (3H, s, H-30), and at 1.73 (3H, s, Me-27). Two double doublet protons at δH 3.30 (1H, dd, J = 4.4, 10.8 Hz, H-3), and δH 4.03 (1H, t, J = 5.8 Hz, H-24) revealed presence of two carbinolic protons and a pair of olefinic protons at δH 4.95 and 4.86 (each 1H, brs, H-26) suggested a terminal methylene. Downfield chemical shift of one singlet methyl proton at δH 1.73 (H-27) of the side chain atoms was in accordance with the quaternary olefinic group on C-25 at δC 128.8. As Ayatollahi and coworkers described EI-MS fragmentation pattern of cycloartanes (4), presence of monounsaturated side chain was also confirmed by the m/z 315 and 297 in EI-MS. In addition, m/z 381 together with 355 [M-H2O-C5H9]+ fragments due to the elimination of parts of side chain during a Mc Lafferty process, inferred presence of one hydroxyl in side-chain. Regarding to these findings, and literature data (4), compound 1 identified as cycloart-25-en-3 β, 24-diol. It is also found in other Euphorbia species like E. aellenii (4), E. heteradena (5) and E. sessiliflora (6).
Compound 2, and 3 showed the molecular formula of C30H50O2 based on positive EI- MS m/z 442 and in accordance with their number and the multiplicity of 13C-NMR spectra (BB and DEPT). Their 1H-NMR revealed six tertiary singlet methyls, one secondary methyl group, and a pair of doublets in the up-field area characteristic of cycloartane cyclopropane ring and one carbinolic proton related to 3(β)-OH group. In compound 2, in olefinic pair protons, δH 4.94 (1H, brs, H-24) showed low coupling constants with at δH 4.96 (1H, m, H-23) due to their cis orientation while in compound 3, olefinic pair protons at δH 5.72 (1H, ddd, J = 15.6, 8.4, 6.0 Hz, H-23) and 5.54 (1H, d, J = 15.6 Hz, H-24) with large coupling constant (J = 15.6 Hz) allowed assignment of trans geometry to the Δ23(24). In both compounds, downfield chemical shifts of two singlet methyl protons (Me-26, and Me-27) of the side chain atoms were in accordance with the second hydroxyl group on C-25 at δC 70.8 and 68.2, respectively. Therefore, based on aforementioned data and complete agreements of 13C- and 1H-NMR with other reported data in literature (7; 8), compound 2 and 3 were identified as cycloart-23Z-ene-3β, 25-diol and cycloart-23E-ene-3β, 25-diol (Figure 1). They are also reported in Euphorbia spinidens (9), E. rigida (10), and E. humifusa (11).
Compound 4, showed the molecular formula of C31H52O based on EI-MS m/z 440 and number and multiplicity of 13C-NMR spectra. The six-degree of unsaturation and the 13C-NMR data (Table 1), suggested the presence of one double bond and consequently five rings in the molecule. The 13C-NMR data (BB and DEPT), encompassed thirty-one carbons.1H-NMR revealed a pair of doublets in the up-field area at δH 0.30 and 0.53 (J = 4.25 Hz) characteristic of cycloartanes, four singlet methyls at δH 0.83 (3H, s, H-29), 0.91 (3H, s, H-28), and 0.99 (2× 3H, s, H-18, H-30) together with three secondary methyls. A doublet of doublet proton at δH 3.31, indicative of a carbinolic group, and one pair of olefinic protons δH 4.74, and 4.69 (each 1H, bs, H-31a, b) related to exocyclic terminal methylene. According to the literarture and these data, compound 4 was determined as 24-methylene-cycloartan-3β-ol (4). It was found in other spurge species like E. rigida (10), and E. aellenii (4).
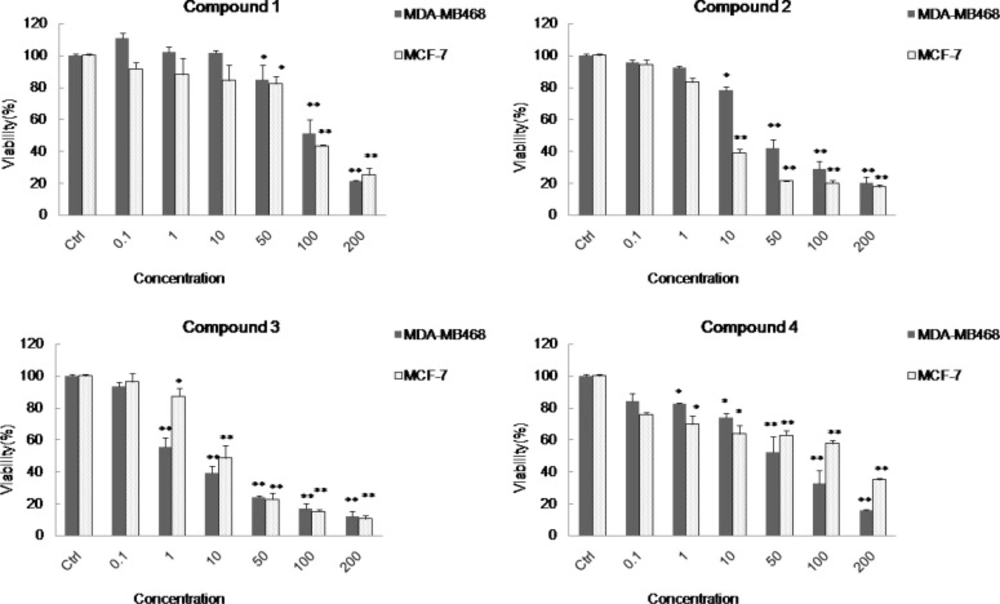
Using MTT assay on two different cancer cell lines (3,12-13), the biological effects of the compounds (1-4) on two different cancer cell lines including MDA-MB48 and MCF-7 showed LD50 values of 102.3, 34.0, 2.05, and 53.8 μgmL−1 on MDA-MB468 cell line, and LD50 values of 88.3, 5.4, 8.9, and 127.3 μgmL− 1 on MCF-7 cell line, respectively. Among these compounds, cycloart-23(E)-ene-3β,25-diol (3) was the most active compound on MDA-MB468 cell line (LD50 = 2.05 μgmL− 1 ) and cycloart-23(Z)-ene-3β,25-diol (2) was the most active compound on MCF-7 cell line (LD50 = 5.4 μgmL− 1 ).
The potent cytotoxicity observed by compound 2 and 3 with double bound on C-23 suggested that the cytotoxicity activities of these compounds are related to the position of the olefinic or the hydroxyl group on side chain.
Cytotoxicity effects of the cycloartanes (1-4) in Euphorbia macrostegia on two cancer cell lines MDA-MB48 and MCF-7 . In this panel the cytotoxicity tests were presented on two different cancer cell lines including MDA-MB48 and MCF-7 in the presence of different concentrations (0.1, 1, 10, 50, 100 and 200 μg/mL) of cycloart-25-ene-3β,24-diol (1), cycloart-23(Z)-ene-3β,25-diol (2), cycloart-23(E)-ene-3β, 25-diol (3), and 24-methylene-cycloart-3β-ol (4), and control cells which were not treated (set to 100%). For statistical significance one-way ANOVA was used to analyze the differences between each sample and control (*P < 0.05, **P < 0.01).
By the literature, cycloartanes isolated from Euphorbia species showed also apoptosis induction on mouse lymphoma cells (14). Cycloart-25-en-3(β), 24-diol and 24-methylene-cycloartan-3(β)-ol (compound 1 and 4) presented antiproliferated activity on human peripheral blood lymphocytes (4). Cycloartanes were also reported for other biological activities like immunomodulatory effects like positive effect on Th1 cytokine release (IL-2 and IFN-γ), and suppression on Th2 cytokine production (IL-4) (15), inhibition of 11β-hydroxysteroid dehydrogenases (11β-HSD1 and 11β-HSD2) as a strategy for reducing glucocorticoid action on insulin resistance in type 2 diabetes mellitus and metabolic syndrome (16,17), or stimulating GLP-1 amide secretion in streptozotocin-nicotinamide induced diabetic Sprague Dawley rats (18). Therefore, interesting properties of cycloartanes, especially their antiproliferative effects, candidate them as investigational lead compounds in cancer research.